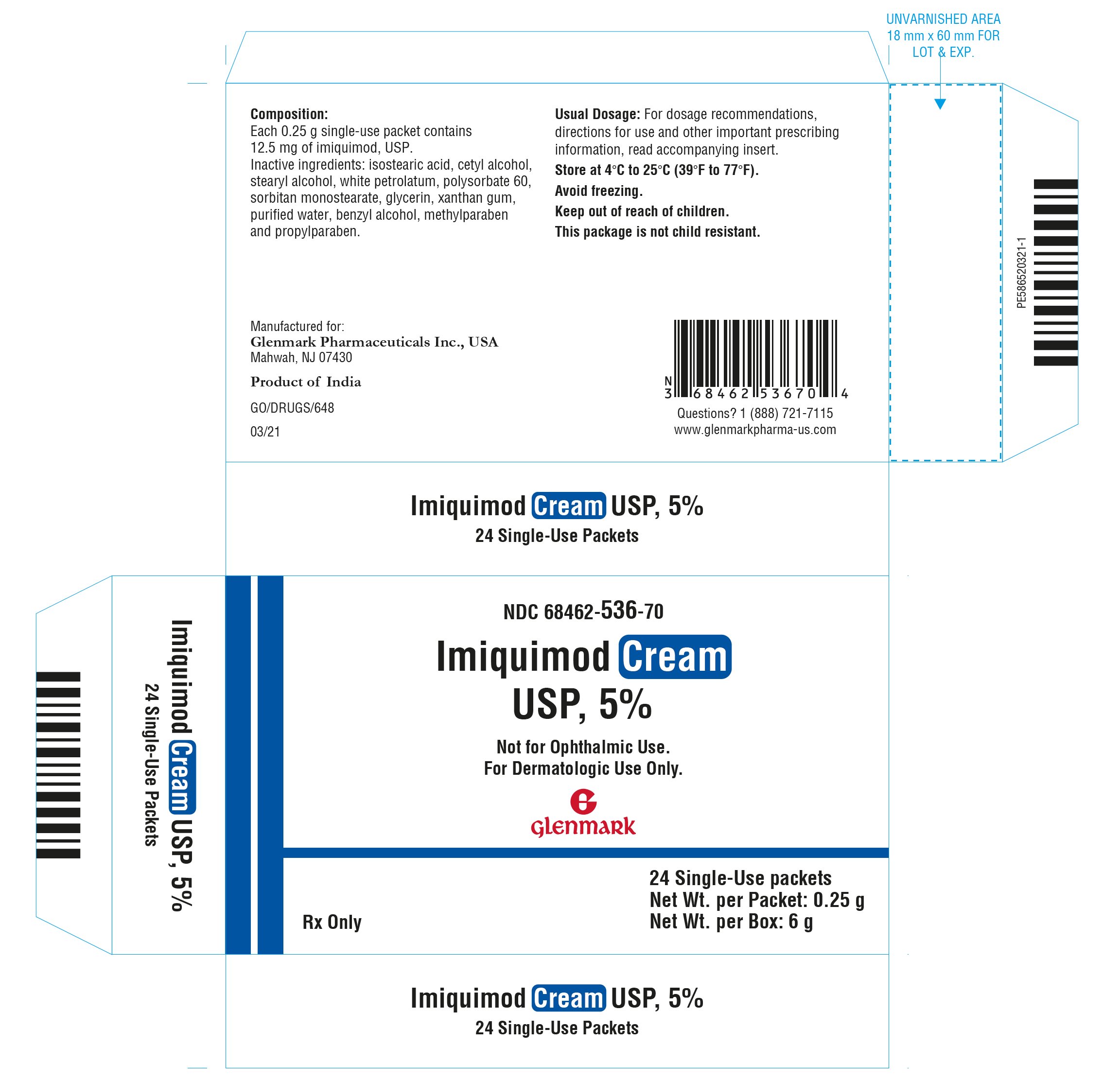
Drug Catalog - Product Detail
IMIQUIMOD CREAM CRM 0.05 BOX-24PCKT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0536-70 | GLENMARK PHARMACEUTICALS | 24 | 5% | CREAM |
PACKAGE FILES

Generic Name
IMIQUIMOD
Substance Name
IMIQUIMOD
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA201994
Description
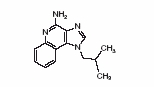
11 DESCRIPTION Imiquimod cream USP is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod, USP in an off-white oil-in-water vanishing cream base consisting of benzyl alcohol, cetyl alcohol, glycerin, isostearic acid, methylparaben, polysorbate 60, propylparaben, purified water, sorbitan monostearate, stearyl alcohol, white petrolatum and xanthan gum. Chemically, imiquimod, USP is 1-(2-methylpropyl)-1 H-imidazo[4,5-c]quinolin-4-amine. Imiquimod, USP has a molecular formula of C 14 H 16 N 4 and a molecular weight of 240.3 g/mol. Its structural formula is: structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Imiquimod Cream USP, 5% is supplied in single-use packets, which contain 250 mg of the cream. Available as: Box of 24 packets NDC 68462-536-70. Store at 4°C to 25°C (39°F to 77°F). Avoid freezing. Keep out of reach of children.
Indications & Usage
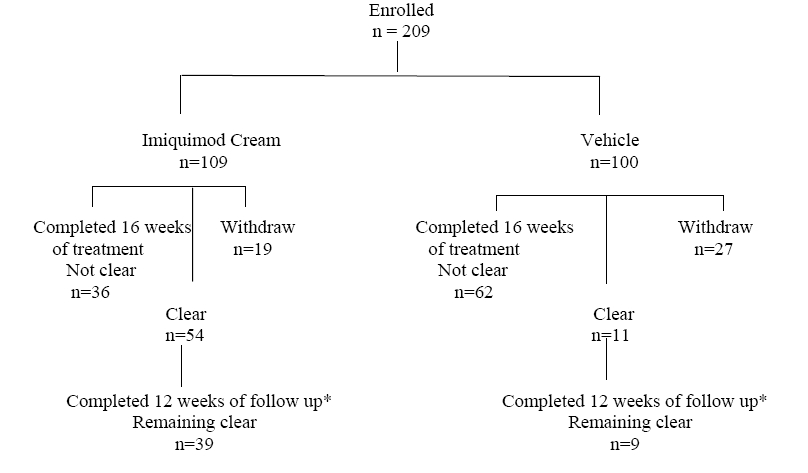
1 INDICATIONS AND USAGE Imiquimod cream is indicated for the topical treatment of: • Clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses (AK) on the face or scalp in immunocompetent adults ( 1.1 ) • External genital and perianal warts/condyloma acuminata in patients 12 years old or older ( 1.3 ) Limitations of Use: Efficacy was not demonstrated for molluscum contagiosum in children aged 2 to12 ( 1.4 , 8.4 ) 1.1 Actinic Keratosis Imiquimod Cream is indicated for the topical treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adults. 1.3 External Genital Warts Imiquimod Cream is indicated for the treatment of external genital and perianal warts/condyloma acuminata in patients 12 years old or older. 1.4 Limitations of Use Imiquimod Cream has been evaluated in children ages 2 to 12 years with molluscum contagiosum and these studies failed to demonstrate efficacy [see Use in Specific Populations (8.4) ] . 1.5 Unevaluated Populations The safety and efficacy of Imiquimod Cream in immunosuppressed patients have not been established. Imiquimod Cream should be used with caution in patients with pre-existing autoimmune conditions. The efficacy and safety of Imiquimod Cream have not been established for patients with Basal Cell Nevus Syndrome or Xeroderma Pigmentosum.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The application frequency for Imiquimod Cream is different for each indication. Imiquimod is not for oral, ophthalmic, or intravaginal use. Imiquimod cream is not for oral, ophthalmic, or intravaginal use ( 2 ) • Actinic keratosis: 2 times per week for a full 16 weeks ( 2.1 ) • External genital warts (EGW): 3 times per week until total clearance or a maximum of 16 weeks ( 2.3 ) 2.1 Actinic Keratosis Imiquimod Cream should be applied 2 times per week for a full 16 weeks to a defined treatment area on the face or scalp (but not both concurrently). The treatment area is defined as one contiguous area of approximately 25 cm 2 (e.g., 5 cm x 5 cm) on the face (e.g. forehead or one cheek) or on the scalp. Examples of 2 times per week application schedules are Monday and Thursday, or Tuesday and Friday. Imiquimod Cream should be applied to the entire treatment area and rubbed in until the cream is no longer visible. No more than one packet of Imiquimod Cream should be applied to the contiguous treatment area at each application. Imiquimod Cream should be applied prior to normal sleeping hours and left on the skin for approximately 8 hours, after which time the cream should be removed by washing the area with mild soap and water . The prescriber should demonstrate the proper application technique to maximize the benefit of Imiquimod Cream therapy. It is recommended that patients wash their hands before and after applying Imiquimod Cream. Before applying the cream, the patient should wash the treatment area with mild soap and water and allow the area to dry thoroughly (at least 10 minutes). Contact with the eyes, lips and nostrils should be avoided. Local skin reactions in the treatment area are common [see Adverse Reactions ( 6.1 , 6.5 )] . A rest period of several days may be taken if required by the patient's discomfort or severity of the local skin reaction. However, the treatment period should not be extended beyond 16 weeks due to missed doses or rest periods . Response to treatment cannot be adequately assessed until resolution of local skin reactions. Lesions that do not respond to treatment should be carefully re-evaluated and management reconsidered. Imiquimod Cream is packaged in single-use packets, with 24 packets supplied per box. Patients should be prescribed no more than 36 packets for the 16-week treatment period. Unused packets should be discarded. Partially-used packets should be discarded and not reused. 2.3 External Genital Warts Imiquimod Cream should be applied 3 times per week to external genital/perianal warts. Imiquimod Cream treatment should continue until there is total clearance of the genital/perianal warts or for a maximum of 16 weeks. Examples of 3 times per week application schedules are: Monday, Wednesday, Friday or Tuesday, Thursday, Saturday. Imiquimod Cream should be applied prior to normal sleeping hours and left on the skin for 6 to 10 hours, after which time the cream should be removed by washing the area with mild soap and water . The prescriber should demonstrate the proper application technique to maximize the benefit of Imiquimod Cream therapy. It is recommended that patients wash their hands before and after applying Imiquimod Cream. A thin layer of Imiquimod Cream should be applied to the wart area and rubbed in until the cream is no longer visible. The application site should not be occluded. Following the treatment period the cream should be removed by washing the treated area with mild soap and water. Local skin reactions at the treatment site are common [see Adverse Reactions ( 6.3 , 6.5 )] . A rest period of several days may be taken if required by the patient's discomfort or severity of the local skin reaction. Treatment may resume once the reaction subsides. Non-occlusive dressings such as cotton gauze or cotton underwear may be used in the management of skin reactions. Imiquimod Cream is packaged in single-use packets which contain sufficient cream to cover a wart area of up to 20 cm 2 ; use of excessive amounts of cream should be avoided.
