Drug Catalog - Product Detail
INDAPAMIDE 2.5MG TAB 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62559-0511-01 | ANI PHARMACEUTICALS | 100 | 2.5MG | TABLET |
PACKAGE FILES



Generic Name
INDAPAMIDE
Substance Name
INDAPAMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA074299
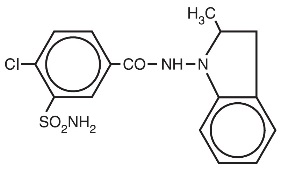
Description
DESCRIPTION Indapamide is an oral antihypertensive/diuretic. Its molecule contains both a polar sulfamoyl chlorobenzamide moiety and a lipid-soluble methylindoline moiety. It differs chemically from the thiazides in that it does not possess the thiazide ring system and contains only one sulfonamide group. The chemical name of indapamide is 4-Chloro- N -(2-methyl-1-indolinyl)-3-sulfamoylbenzamide, and its molecular weight is 365.84. The compound is a weak acid, pK a =8.8, and is soluble in aqueous solutions of strong bases. It is a white to yellow-white crystalline (tetragonal) powder. C 16 H 16 ClN 3 O 3 S Each tablet, for oral administration, contains 1.25 mg or 2.5 mg of indapamide USP and the following inactive ingredients: corn starch, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, talc, and titanium dioxide. Additionally, the 1.25 mg product contains FD&C yellow #6 aluminum lake. structure
How Supplied
HOW SUPPLIED Indapamide Tablets USP are available containing 1.25 mg or 2.5 mg of indapamide USP. The 1.25 mg tablets are orange, round, film coated tablets debossed ‘ANI’ over ‘510’ on one side and plain on the other side. They are available in bottles of 100 tablets (NDC 62559-510-01). The 2.5 mg tablets are white, round, film coated tablets debossed ‘ANI’ over ‘511’ on one side and plain on the other side. They are available in bottles of 100 tablets (NDC 62559-511-01). Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Avoid excessive heat. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. Manufactured by: ANI Pharmaceuticals, Inc. Baudette, MN 56623 9957 Rev 03/21 ani
Indications & Usage
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension The adult starting indapamide dose for hypertension is 1.25 mg as a single daily dose taken in the morning. If the response to 1.25 mg is not satisfactory after 4 weeks, the daily dose may be increased to 2.5 mg taken once daily. If the response to 2.5 mg is not satisfactory after 4 weeks, the daily dose may be increased to 5 mg taken once daily, but adding another antihypertensive should be considered. Edema of Congestive Heart Failure The adult starting indapamide dose for edema of congestive heart failure is 2.5 mg as a single daily dose taken in the morning. If the response to 2.5 mg is not satisfactory after one week, the daily dose may be increased to 5 mg taken once daily. If the antihypertensive response to indapamide is insufficient, indapamide may be combined with other antihypertensive drugs, with careful monitoring of blood pressure. It is recommended that the usual dose of other agents be reduced by 50% during initial combination therapy. As the blood pressure response becomes evident, further dosage adjustments may be necessary. In general, doses of 5 mg and larger have not appeared to provide additional effects on blood pressure or heart failure, but are associated with a greater degree of hypokalemia. There is minimal clinical trial experience in patients with doses greater than 5 mg once a day.
