Drug Catalog - Product Detail
INVOKAMET TABS 150-1000MG 60CT CPLT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 50458-0543-60 | JANSSEN | 60 | 150-1000MG | TABLET |
PACKAGE FILES










Generic Name
CANAGLIFLOZIN AND METFORMIN HYDROCHLORIDE
Substance Name
CANAGLIFLOZIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA204353
Description
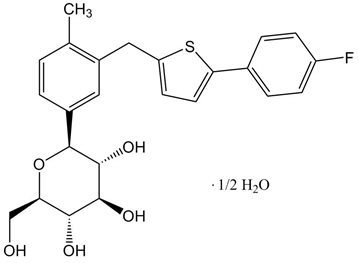
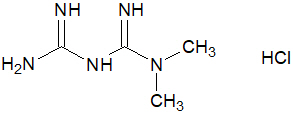
11 DESCRIPTION INVOKAMET ® (canagliflozin and metformin HCl immediate-release tablets) and INVOKAMET ® XR (canagliflozin and metformin HCl extended-release tablets) contain canagliflozin and metformin HCl. Canagliflozin Canagliflozin is an inhibitor of SGLT2, the transporter responsible for reabsorbing the majority of glucose filtered by the kidney. Canagliflozin is chemically known as (1 S )-1,5-anhydro-1-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol hemihydrate and its molecular formula and weight are C 24 H 25 FO 5 S•1/2 H 2 O and 453.53, respectively. The structural formula for canagliflozin is: Canagliflozin is practically insoluble in aqueous media from pH 1.1 to 12.9. Chemical Structure Metformin HCl Metformin HCl is a biguanide chemically known as 1,1-Dimethylbiguanide HCl and its molecular formula and weight are C 4 H 11 N 5 ● HCl and 165.62, respectively. The structural formula for metformin HCl is: Chemical Structure INVOKAMET and INVOKAMET XR INVOKAMET or INVOKAMET XR are supplied as film-coated tablets for oral administration. Each 50 mg/500 mg tablet and 50 mg/1,000 mg tablet contains 51 mg of canagliflozin equivalent to 50 mg canagliflozin (anhydrous) and 500 mg or 1,000 mg metformin HCl (equivalent to metformin 389.93 mg and 779.86 mg, respectively). Each 150 mg/500 mg tablet and 150 mg/1,000 mg tablet contains 153 mg of canagliflozin equivalent to 150 mg canagliflozin (anhydrous) and 500 mg or 1,000 mg metformin HCl (equivalent to metformin 389.93 mg and 779.86 mg, respectively). INVOKAMET contains the following inactive ingredients: croscarmellose sodium (E468), hypromellose, magnesium stearate (E572), and microcrystalline cellulose (E460[i]). The magnesium stearate is vegetable-sourced. The tablets are finished with a commercially available film-coating consisting of the following inactive ingredients: macrogol/PEG3350 (E1521), polyvinyl alcohol (E1203) (partially hydrolyzed), talc (E553b), titanium dioxide (E171), iron oxide yellow (E172) (50 mg/1,000 mg and 150 mg/500 mg tablets only), iron oxide red (E172) (50 mg/1,000 mg, 150 mg/500 mg and 150 mg/1,000 mg tablets only), and iron oxide black (E172) (150 mg/1,000 mg tablets only). INVOKAMET XR contains the following inactive ingredients: croscarmellose sodium (E468), hydroxypropyl cellulose (E463), hypromellose, lactose anhydrous, magnesium stearate (E572) (vegetable-sourced), microcrystalline cellulose (E460[i]), polyethylene oxide, and silicified microcrystalline cellulose (50 mg/500 mg and 50 mg/1,000 mg tablets only). The tablets are finished with a commercially available film-coating consisting of the following inactive ingredients: macrogol/PEG3350 (E1521), polyvinyl alcohol (E1203) (partially hydrolyzed), talc (E553b), titanium dioxide (E171), iron oxide red (E172), iron oxide yellow (E172), and iron oxide black (E172) (50 mg/1,000 mg and 150 mg/1,000 mg tablets only). INVOKAMET XR tablets provide canagliflozin for immediate-release and metformin HCl for extended-release. Each bilayer tablet is compressed from two separate granulates, one for each active ingredient of the tablet, and finished with a film-coating. The metformin HCl extended-release layer is based on a polymer matrix which controls the drug release by passive diffusion through the swollen matrix in combination with tablet erosion.
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING INVOKAMET ® tablets are available in bottles of 60 in the strengths listed below: INVOKAMET TABLET STRENGTH canagliflozin/metformin HCl tablets 50 mg/500 mg 50 mg/1,000 mg 150 mg/500 mg 150 mg/1,000 mg Color White Beige Yellow Purple Tablet Identification CM CM CM CM 155 551 215 611 Capsule-shaped, film-coated tablets NDC 50458-540-60 50458-541-60 50458-542-60 50458-543-60 INVOKAMET ® XR tablets are available in bottles of 60 in the strengths listed below: INVOKAMET XR TABLET STRENGTH canagliflozin/metformin HCl extended-release tablets 50 mg/500 mg 50 mg/1,000 mg 150 mg/500 mg 150 mg/1,000 mg Color Almost White to Light Orange Pink Orange Reddish Brown Tablet Identification CM1 CM3 CM2 CM4 Oblong, biconvex, film-coated tablets, a thin line on the tablet side may be visible. NDC 50458-940-01 50458-941-01 50458-942-01 50458-943-01 Storage and Handling Keep out of reach of children. Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30°C (59 °F to 86 °F) [see USP Controlled Room Temperature] . Store and dispense in the original container. Storage in a pill box or pill organizer is allowed for up to 30 days.
Indications & Usage
1 INDICATIONS AND USAGE INVOKAMET and INVOKAMET XR are a combination of canagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, and metformin hydrochloride (HCl), a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus ( 1 ). Canagliflozin Canagliflozin, when used as a component of INVOKAMET or INVOKAMET XR is indicated in adults with type 2 diabetes mellitus to reduce the risk of: Major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease ( 1 ). End-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria ( 1 ). Limitations of Use: Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus ( 1 ). INVOKAMET INVOKAMET is a combination of canagliflozin and metformin HCl immediate-release indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus. INVOKAMET XR INVOKAMET XR is a combination of canagliflozin and metformin HCl extended-release indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus. Canagliflozin Canagliflozin, when used as a component of INVOKAMET or INVOKAMET XR, is indicated in adults with type 2 diabetes mellitus to reduce the risk of: Major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD). End-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular (CV) death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria greater than 300 mg/day. Limitations of Use INVOKAMET or INVOKAMET XR are not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus [see Warnings and Precautions (5.2) ] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Assess renal function before initiating and as clinically indicated. Assess volume status and correct volume depletion before initiating ( 2.1 ). Individualize starting dose based on the patient's current regimen and renal function. See Table 1 in the full prescribing information for recommended starting dosages based on the current regimen ( 2.2 , 2.3 ). The maximum recommended total daily dosage is 300 mg of canagliflozin and 2,000 mg of metformin HCl ( 2.2 ). Initiation of INVOKAMET or INVOKAMET XR is not recommended in patients with an eGFR less than 45 mL/min/1.73 m 2 , due to the metformin HCl component ( 2.3 ). INVOKAMET: take one tablet orally twice daily with meals ( 2.2 ). INVOKAMET XR: take two tablets orally once daily with the morning meal. Swallow whole. Never crush, cut, or chew ( 2.2 ). Gradually escalate the dosage of metformin HCl in INVOKAMET or INVOKAMET XR to reduce the risk of gastrointestinal adverse reactions with metformin HCl ( 2.2 ). Dose adjustment for patients with renal impairment may be required ( 2.3 ). See full prescribing information for INVOKAMET and INVOKAMET XR dosage modifications due to drug interactions ( 2.4 ). May need to be discontinued at time of, or prior to, iodinated contrast imaging procedures ( 2.5 ). Withhold INVOKAMET or INVOKAMET XR at least 3 days, if possible, prior to surgery or procedures associated with prolonged fasting ( 2.6 ). 2.1 Prior to Initiation of INVOKAMET or INVOKAMET XR Assess renal function before initiating INVOKAMET or INVOKAMET XR and as clinically indicated [see Dosage and Administration (2.3) , Contraindications (4) , and Warnings and Precautions (5.1 , 5.4) ]. In patients with volume depletion, correct this condition before initiating INVOKAMET or INVOKAMET XR [see Warnings and Precautions (5.4) and Use in Specific Populations (8.5 , 8.6) ] . 2.2 Recommended Dosage and Administration INVOKAMET and INVOKAMET XR INVOKAMET and INVOKAMET XR contain canagliflozin and metformin HCl. For the available strengths of the canagliflozin and metformin HCl components in INVOKAMET and INVOKAMET XR, see Dosage Forms and Strengths (3) . Individualize the starting dosage of INVOKAMET or INVOKAMET XR based on the patient's current regimen as presented in Table 1 and based on renal function as presented in Table 2 [see Dosage and Administration (2.3 ] . INVOKAMET Take one tablet of INVOKAMET orally twice daily with meals. INVOKAMET XR Take two tablets of INVOKAMET XR orally once daily with the morning meal. Swallow each tablet whole and never crush, cut, or chew. Table 1 presents the recommended starting dosage of INVOKAMET and INVOKAMET XR based on the patient's current regimen. Table 1: Recommended Starting Dosage Based on the Current Regimen Current Regimen INVOKAMET Recommended Dosage INVOKAMET XR Recommended Dosage Not treated with either canagliflozin or metformin HCl Total daily dosage is canagliflozin 100 mg and metformin HCl 1,000 mg Metformin HCl For patients taking an evening dosage of metformin HCl extended-release tablets, skip the last dose before starting INVOKAMET or INVOKAMET XR the following morning. Total daily dosage is canagliflozin 100 mg and the nearest appropriate total daily dosage of metformin HCl Canagliflozin The same total daily dosage of canagliflozin and a total daily dosage of metformin HCl 1,000 mg Canagliflozin and metformin HCl The same total daily dosage of canagliflozin and the nearest appropriate total daily dosage of metformin HCl Recommended Dosage for Additional Glycemic Control in Adults and Pediatric Patients Aged 10 Years and Older INVOKAMET The dosage of canagliflozin in INVOKAMET may be increased to the maximum total daily dosage of 300 mg (150 mg orally twice daily) in patients tolerating a dosage of 100 mg (50 mg twice daily) of canagliflozin. The dosage of metformin HCl in INVOKAMET may be increased to the maximum total daily dosage of 2,000 mg (1,000 mg orally twice daily), with gradual escalation to reduce the risk of gastrointestinal adverse reactions with metformin HCl [see Adverse Reactions (6.1) ]. INVOKAMET XR The dosage of canagliflozin in INVOKAMET XR may be increased to the maximum total daily dosage of 300 mg orally once daily in patients tolerating a 100 mg once daily dosage of canagliflozin. The dosage of metformin HCl in INVOKAMET XR may be increased to the maximum total daily dosage of 2,000 mg once daily, with gradual escalation to reduce the risk of gastrointestinal adverse reactions with metformin HCl [see Adverse Reactions (6.1) ]. 2.3 Recommended Dosage in Adults and Pediatric Patients Aged 10 Years and Older with Renal Impairment Initiation of INVOKAMET or INVOKAMET XR is not recommended in adults or pediatric patients aged 10 years and older with an eGFR less than 45 mL/min/1.73 m 2 , due to the metformin component. Table 2 provides dosage recommendations for adults and pediatric patients aged 10 years and older with renal impairment, based on eGFR [see Use in Specific Populations (8.6) and Clinical Studies (14.4) ]. Table 2: Recommended Dosage in Adults and Pediatric Patients Aged 10 Years and Older with Renal Impairment Estimated Glomerular Filtration Rate [eGFR (mL/min/1.73 m 2 )] Recommended Dosage of INVOKAMET or INVOKAMET XR For the dosing frequency of INVOKAMET and INVOKAMET XR, see Dosage and Administration (2.2). eGFR 45 to less than 60 The maximum total daily dosage of canagliflozin is 100 mg. eGFR 30 to less than 45 Assess the benefit risk of continuing INVOKAMET or INVOKAMET XR. The maximum total daily dosage of canagliflozin is 100 mg. eGFR less than 30 Contraindicated. If eGFR falls below 30 during treatment; discontinue INVOKAMET or INVOKAMET XR [see Contraindications (4) ] . 2.4 Concomitant Use with UDP-Glucuronosyltransferase (UGT) Enzyme Inducers When co-administering INVOKAMET or INVOKAMET XR with an inducer of UGT (e.g., rifampin, phenytoin, phenobarbital, ritonavir), increase the total daily dosage of canagliflozin based on renal function [see Drug Interactions (7) ] : In patients with eGFR 60 mL/min/1.73 m 2 or greater, increase the total daily dosage of canagliflozin to 200 mg in patients currently tolerating a total daily dosage of canagliflozin 100 mg. The maximum total daily dosage of canagliflozin is 300 mg. In patients with eGFR less than 60 mL/min/1.73 m 2 , increase the total daily dosage of canagliflozin to a maximum of 200 mg in patients currently tolerating a total daily dosage of canagliflozin 100 mg. 2.5 Discontinuation for Iodinated Contrast Imaging Procedures Discontinue INVOKAMET or INVOKAMET XR at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR of less than 60 mL/min/1.73 m 2 ; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart INVOKAMET or INVOKAMET XR if renal function is stable [see Warnings and Precautions (5.1) ] . 2.6 Temporary Interruption for Surgery Withhold INVOKAMET or INVOKAMET XR at least 3 days, if possible, prior to surgery or procedures associated with prolonged fasting. Resume INVOKAMET or INVOKAMET XR when the patient is clinically stable and has resumed oral intake [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2) ].
