Drug Catalog - Product Detail
IPRATROPIUM BROMIDE/ALBUTEROL SULFATE INHALATION SOLUTION SOL 0.5/3MG 30X3ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-9671-30 | MYLAN | 3 | 0.5-2.5 (3)MG/3ML | SOLUTION |
PACKAGE FILES

Generic Name
IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE
Substance Name
ALBUTEROL SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
ANDA202496
Description
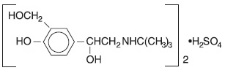
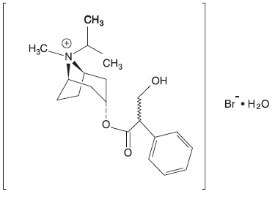
DESCRIPTION The active components in Ipratropium Bromide and Albuterol Sulfate Inhalation Solution are albuterol sulfate and ipratropium bromide. Albuterol sulfate, is a salt of racemic albuterol and a relatively selective β -adrenergic bronchodilator chemically described as α 1 -[( tert -butylamino) methyl]-4- hydroxy - m -xylene-α, α'-diol sulfate (2:1) (salt). It has a molecular weight of 576.7 and the empirical formula is (C 13 H 21 NO 3 ) 2 •H 2 SO 4 . It is a white crystalline powder, soluble in water and slightly soluble in ethanol. The World Health Organization recommended name for albuterol base is salbutamol. Figure 3.1-1. Chemical structure of albuterol sulfate. Ipratropium bromide is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo [3.2.1]-octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8methyl-8-(1-methylethyl)-, bromide, monohydrate ( endo , syn )-, (±)-; a synthetic quaternary ammonium compound, chemically related to atropine. It has a molecular weight of 430.4 and the empirical formula is C 20 H 30 BrNO 3 •H 2 O. It is a white crystalline substance, freely soluble in water and lower alcohols, and insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons. Figure 3.1-2. Chemical structure of ipratropium bromide. Each 3 mL Sterile Unit-dose Vial contains 0.5 mg of ipratropium bromide (0.017%) and 3 mg Equivalent to 2.5 mg albuterol base albuterol sulfate (0.083%) in an isotonic, sterile, aqueous solution containing sodium chloride and 1 N hydrochloric acid to adjust to pH 4. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is a clear, colorless solution. It does not require dilution prior to administration by nebulization. For Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the jet nebulizer utilized, and compressor performance. Using the Pari-LC-Plus™ nebulizer (with face mask or mouthpiece) connected to a PRONEB™ compressor system, under in vitro conditions, the mean delivered dose from the mouth piece (% nominal dose) was approximately 46% of albuterol and 42% of ipratropium bromide at a mean flow rate of 3.6 L/min. The mean nebulization time was 15 minutes or less. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered from jet nebulizers at adequate flow rates, via face masks or mouthpieces (see DOSAGE AND ADMINISTRATION ). Figure 3.1-1. Chemical structure of albuterol sulfate Figure 3.1-2. Chemical structure of ipratropium bromide.
How Supplied
HOW SUPPLIED Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is supplied as a 3-mL sterile solution for nebulization in sterile low-density polyethylene unit-dose vials. Store in pouch until time of use. Supplied in cartons as listed below. NDC 0378-9671-93 30 vials per carton / 1 vial per foil pouch NDC 0378-9671-58 30 vials per carton / 5 vials per foil pouch NDC 0378-9671-30 30 vials per carton / 30 vials per foil pouch NDC 0378-9671-91 60 vials per carton / 5 vials per foil pouch NDC 0378-9671-60 60 vials per carton / 30 vials per foil pouch Store between 2°C and 25°C (36°F and 77°F). Protect from light.
Indications & Usage
INDICATIONS AND USAGE Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is indicated for the treatment of bronchospasm associated with COPD in patients requiring more than one bronchodilator.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended dose of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is one 3 mL vial administered 4 times per day via nebulization with up to 2 additional 3 mL doses allowed per day, if needed. Safety and efficacy of additional doses or increased frequency of administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution beyond these guidelines has not been studied and the safety and efficacy of extra doses of albuterol sulfate or ipratropium bromide in addition to the recommended doses of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution have not been studied. The use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution can be continued as medically indicated to control recurring bouts of bronchospasm. If a previously effective regimen fails to provide the usual relief, medical advice should be sought immediately, as this is often a sign of worsening COPD, which would require reassessment of therapy. A Pari-LC-Plus™ nebulizer (with face mask or mouthpiece) connected to a PRONEB™ compressor was used to deliver Ipratropium Bromide and Albuterol Sulfate Inhalation Solution to each patient in one U.S. clinical study. The safety and efficacy of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution delivered by other nebulizers and compressors have not been established. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered via jet nebulizer connected to an air compressor with an adequate air flow, equipped with a mouthpiece or suitable face mask.
