Drug Catalog - Product Detail
ISOSORBIDE MONONITRATE ER TB 30MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62175-0128-37 | KREMERS URBAN | 100 | 30MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
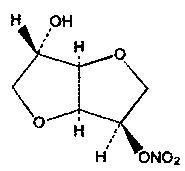
DESCRIPTION Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins. Each tablet, for oral administration, contains either 30 mg, 60 mg or 120 mg of isosorbide mononitrate in an extended-release formulation. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, hydrogenated castor oil, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose and talc. The molecular formula of ISMN is C 6 H 9 NO 6 and the molecular weight is 191.14. The chemical name for ISMN is 1,4:3,6-dianhydro-,D-glucitol 5-nitrate; the compound has the following structural formula: ISMN is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of about 90°C, and an optical rotation of +144° (2% in water, 20°C). Isosorbide mononitrate is freely soluble in water, ethanol, methanol, chloroform, ethyl acetate, and dichloromethane. Structural Formula
How Supplied
HOW SUPPLIED Repackaged by Aphena Pharma Solutions - TN. See Repackaging Information for available configurations. Isosorbide Mononitrate Extended-Release Tablets, USP 30 mg are white, capsule-shaped tablets scored on one side and engraved "KU 128" on the unscored side. They are supplied as follows: Bottles of 90 NDC 62175-128-46 Bottles of 100 NDC 62175-128-37 Bottles of 500 NDC 62175-128-41 Bottles of 1000 NDC 62175-128-43 Isosorbide Mononitrate Extended-Release Tablets, USP 60 mg are white, capsule-shaped tablets scored on one side and engraved "KU 119" on the unscored side. They are supplied as follows: Bottles of 90 NDC 62175-119-46 Bottles of 100 NDC 62175-119-37 Bottles of 500 NDC 62175-128-41 Bottles of 1000 NDC 62175-119-43 Isosorbide Mononitrate Extended-Release Tablets, USP 120 mg are white, capsule-shaped tablets engraved "KU 129" on one side. They are supplied as follows: Bottles of 100 NDC 62175-129-37 Bottles of 1000 NDC 62175-129-43 Store at 20°-30°C (68°-86°F) [See USP]. Distributed by: Kremers Urban Pharmaceuticals Inc. Princeton, NJ 08540, USA L3958L Rev. 4E 01/2011
Indications & Usage
INDICATIONS AND USAGE Isosorbide Mononitrate Extended-Release Tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of oral isosorbide mononitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended starting dose of Isosorbide Mononitrate Extended-Release Tablets is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once daily. After several days, the dosage may be increased to 120 mg (given as a single 120 mg tablet or as two 60 mg tablets) once daily. Rarely, 240 mg may be required. The daily dose of Isosorbide Mononitrate Extended-Release Tablets should be taken in the morning on arising. Isosorbide Mononitrate Extended-Release Tablets should not be chewed or crushed and should be swallowed together with a half-glassful of fluid. Do not break the 30 mg tablet.
