Drug Catalog - Product Detail
ISRADIPINE CP 5MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16252-0540-01 | ACTAVIS PHARMA | 100 | 5MG | CAPSULE |
PACKAGE FILES


Generic Name
ISRADIPINE
Substance Name
ISRADIPINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077317
Description
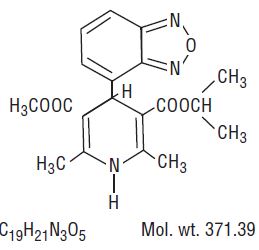
DESCRIPTION Isradipine is a calcium antagonist available for oral administration in capsules containing 2.5 mg or 5 mg. The structural formula of isradipine is: Chemically, isradipine is 3,5-Pyridinedicarboxylic acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, methyl 1-methylethyl ester. Isradipine is a yellow, fine crystalline powder which is odorless or has a faint characteristic odor. Isradipine is practically insoluble in water (<10 mg/L at 37ºC), but is soluble in ethanol and freely soluble in acetone, chloroform and methylene chloride. Active Ingredient: isradipine Inactive Ingredients: colloidal silicon dioxide, red iron oxide (2.5 mg capsule only), yellow iron oxide, gelatin, anhydrous lactose, magnesium stearate, sodium lauryl sulfate, starch (corn), titanium dioxide, black ink: black iron oxide, shellac, potassium hydroxide and propylene glycol. formula
How Supplied
HOW SUPPLIED Isradipine Capsules, USP 2.5 mg Brown opaque, imprinted with “ ” IS 2.5. Bottles of 100 capsules (NDC 16252-539-01) 5 mg Caramel opaque, imprinted with “ ” IS 5. Bottles of 100 capsules (NDC 16252-540-01) Store and Dispense Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] and dispense in a tight, light-resistant container. Manufactured For: Teva Pharmaceuticals Parsippany, NJ 07054 Rev. A 11/2023 Cobalt symbol Cobalt symbol
Indications & Usage
INDICATIONS AND USAGE Hypertension Isradipine is indicated in the management of hypertension. It may be used alone or concurrently with thiazide-type diuretics.
Dosage and Administration
DOSAGE AND ADMINISTRATION The dosage of isradipine should be individualized. The recommended initial dose of isradipine is 2.5 mg b.i.d. alone or in combination with a thiazide diuretic. An antihypertensive response usually occurs within 2 to 3 hours. Maximal response may require 2 to 4 weeks. If a satisfactory reduction in blood pressure does not occur after this period, the dose may be adjusted in increments of 5 mg/day at 2 to 4 week intervals up to a maximum of 20 mg/day. Most patients, however, show no additional response to doses above 10 mg/day, and adverse effects are increased in frequency above 10 mg/day. The bioavailability of isradipine (increased AUC) is increased in elderly patients (above 65 years of age), patients with hepatic functional impairment, and patients with mild renal impairment. Ordinarily, the starting dose should still be 2.5 mg b.i.d. in these patients.
