Drug Catalog - Product Detail
Ketoconazole Tab 200 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 35573-0433-02 | BUREL PHARMACEUTICALS | 100 | 200MG | TABLET |
PACKAGE FILES

Generic Name
KETOCONAZOLE
Substance Name
KETOCONAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075912
Description
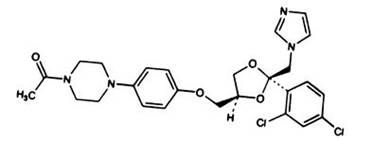
DESCRIPTION Ketoconazole tablets USP is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and methylcellulose. Ketoconazole is cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl] methoxyl]phenyl] piperazine and has the following structural formula: Ketoconazole is a white to slightly beige, odorless powder, soluble in acids, with a molecular weight of 531.44g/mol. FDA approved dissolution test specifications differ from USP. Image
How Supplied
HOW SUPPLIED Ketoconazole tablets USP, 200 mg are white to off-white, round, flat faced bevel edge, scored tablets, debossed "A" over "35" on one side and plain with bisect on the other side. They are supplied in bottles of 30 tablets (NDC 35573-433-30) and 100 tablets (NDC 35573-433-02) with a child-resistant cap. Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. Keep out of reach of children. Dispense with medication guide available at: https://www.burelpharma.com Manufactured by: Aavis Pharmaceuticals, Hoschton, GA 30548 Manufactured for: Burel Pharmaceuticals, LLC Mason, OH 45040 USA Code. L7019/04 Rev. 08/2023
Indications & Usage
INDICATIONS AND USAGE Ketoconazole tablets are not indicated for treatment of onychomycosis, cutaneous dermatophyte infections, or Candida infections. Ketoconazole tablets should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks. Ketoconazole tablets are indicated for the treatment of the following systemic fungal infections in patients who have failed or who are intolerant to other therapies: blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. Ketoconazole tablets should not be used for fungal meningitis because it penetrates poorly into the cerebrospinal fluid.
Dosage and Administration
DOSAGE AND ADMINISTRATION There should be laboratory as well as clinical documentation of infection prior to starting ketoconazole therapy. The usual duration of therapy for systemic infection is 6 months. Treatment should be continued until active fungal infection has subsided. Adults The recommended starting dose of ketoconazole tablets is a single daily administration of 200 mg (one tablet). If clinical responsiveness is insufficient within the expected time, the dose of ketoconazole tablets may be increased to 400 mg (two tablets) once daily. Children In small numbers of children over 2 years of age, a single daily dose of 3.3 to 6.6 mg/kg has been used. Ketoconazole tablets have not been studied in children under 2 years of age.
