Drug Catalog - Product Detail
KETOROLAC TROMETHAMINE OPHTH. SOL. SOL 0.005 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 17478-0209-11 | AKORN | 10 | 0.5% | SOLUTION |
PACKAGE FILES
Generic Name
Substance Name
Product Type
Route
Application Number
Description
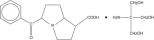
11 DESCRIPTION Ketorolac tromethamine ophthalmic solution, 0.5% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-benzoyl-2, 3-dihydro-1H pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) and it has the following structural formula: The molecular formula of ketorolac tromethamine ophthalmic solution, 0.5% is C 15 H 13 NO 3 •C 4 H 11 NO 3 . Ketorolac tromethamine ophthalmic solution is supplied as a sterile isotonic aqueous 0.5% solution, with a pH of 7.4. Ketorolac tromethamine ophthalmic solution is a racemic mixture of R-(+) and S-(-)- ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The molecular weight of ketorolac tromethamine is 376.41. The osmolality of ketorolac tromethamine ophthalmic solution is 290 mOsmol/kg. Each mL of ketorolac tromethamine ophthalmic solution, 0.5% contains: Active: Ketorolac Tromethamine 0.5%. Inactives: Edetate Disodium 0.1%; Octoxynol 40; Sodium Chloride; Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (6.8 to 7.4); Water for Injection. Preservative: Benzalkonium Chloride 0.01%. Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ketorolac tromethamine ophthalmic solution, 0.5% is supplied sterile in white LDPE plastic bottles with natural droppers and gray short skirt caps as follows: 3 mL in 10 mL bottle - NDC 17478-209-19 5 mL in 10 mL bottle - NDC 17478-209-10 10 mL in 10 mL bottle - NDC 17478-209-11 Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Retain in carton until time of use.
Indications & Usage
1 INDICATIONS AND USAGE Ketorolac tromethamine ophthalmic solution is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. Ketorolac tromethamine ophthalmic solution is also indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction. Ketorolac tromethamine ophthalmic solution is a nonsteroidal, anti-inflammatory indicated for: The treatment of inflammation following cataract surgery. ( 1 ) The temporary relief of ocular itching due to seasonal allergic conjunctivitis. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION One drop of ketorolac tromethamine ophthalmic solution should be applied to the affected eye(s) four times a day for relief of ocular itching due to seasonal allergic conjunctivitis. For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ketorolac tromethamine ophthalmic solution should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period. ( 2.1 ) 2.1 Recommended Dosing Patient Dosing The recommended dose of ketorolac tromethamine ophthalmic solution is one drop four times a day to the affected eye(s) for relief of ocular itching due to seasonal allergic conjunctivitis. For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ketorolac tromethamine ophthalmic solution should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period. 2.2 Use with Other Topical Ophthalmic Medications Ketorolac tromethamine ophthalmic solution has been safely administered in conjunction with other ophthalmic medications such as antibiotics, alpha-agonists, beta blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics. Drops should be administered at least 5 minutes apart.
