Drug Catalog - Product Detail
LEVALBUTEROL TARTRATE HFA INHALATION SOLUTION SOL 45MCG 15GM/200MD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-2927-54 | ACTAVIS PHARMA | 15 | 45MCG/ACT | AEROSOL |
PACKAGE FILES















Generic Name
LEVALBUTEROL TARTRATE
Substance Name
LEVALBUTEROL TARTRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA021730
Description
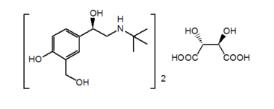
11 DESCRIPTION The active component of Levalbuterol tartrate HFA inhalation aerosol is levalbuterol tartrate, the (R)-enantiomer of albuterol. Levalbuterol tartrate is a relatively selective beta 2 -adrenergic receptor agonist [see Clinical Pharmacology (12) ]. Levalbuterol tartrate has the chemical name (R)-α 1 -[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol L-tartrate (2:1 salt), and it has the following chemical structure: The molecular weight of levalbuterol tartrate is 628.71, and its empirical formula is (C 13 H 21 NO 3 ) 2 · C 4 H 6 O 6 . It is a white to light-yellow solid, freely soluble in water and very slightly soluble in ethanol. Levalbuterol tartrate is the generic name for (R)-albuterol tartrate in the United States. Levalbuterol tartrate HFA inhalation aerosol is a pressurized metered-dose aerosol inhaler (MDI) fitted with a dose indicator, which produces an aerosol for oral inhalation. It contains a suspension of micronized levalbuterol tartrate, propellant HFA-134a (1,1,1,2-tetrafluoroethane), Dehydrated Alcohol USP, and Oleic Acid NF. After priming with 4 actuations, each actuation of the inhaler delivers 67.8 mcg of levalbuterol tartrate (equivalent to 51.6 mcg of levalbuterol free base) from the valve and 59 mcg of levalbuterol tartrate (equivalent to 45 mcg of levalbuterol free base) from the actuator mouthpiece. Each 15 g canister provides 200 actuations (or inhalations). The following chemical structure Levalbuterol tartrate is a relatively selective beta2-adrenergic receptor agonist [see Clinical Pharmacology (12)]. Levalbuterol tartrate has the chemical name (R)-α1-[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol L-tartrate (2:1 salt).
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Levalbuterol tartrate HFA inhalation aerosol is supplied as a pressurized aluminum canister in a box: NDC 0591-2927-54: Canister labeled with a net weight of 15 grams containing 200 metered actuations (or inhalations) Each canister is fitted with a dose indicator and is supplied with a blue plastic actuator mouthpiece, a red mouthpiece cap, and patient’s instructions. Shake well before using . Store between 20° and 25°C (68° and 77°F; see USP controlled room temperature). Protect from freezing temperatures and direct sunlight. Store inhaler with the actuator mouthpiece down. Contents under pressure Do not puncture or incinerate. Do not store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator. Keep out of reach of children. The blue actuator supplied with Levalbuterol tartrate HFA inhalation aerosol should not be used with any other product canisters. Actuators from other products should not be used with a Levalbuterol tartrate HFA inhalation aerosol canister. The correct amount of medication in each actuation cannot be assured after 200 actuations, even though the canister is not completely empty. When the dose indicator display window shows a red zone, approximately 20 inhalations are left, and a refill is required. The canister should be discarded when the dose indicator display window shows zero, indicating that 200 actuations have been used.
Indications & Usage
1 INDICATIONS AND USAGE Levalbuterol tartrate HFA inhalation aerosol is a beta 2 -adrenergic agonist indicated for the treatment or prevention of bronchospasm in patients 4 years of age and older with reversible obstructive airway disease. ( 1.1 ) 1.1 Bronchospasm Levalbuterol tartrate HFA inhalation aerosol is indicated for the treatment or prevention of bronchospasm in adults, adolescents, and children 4 years of age and older with reversible obstructive airway disease.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION For Oral Inhalation Only ( 2.2 ) Adults and children 4 years of age and older: 2 inhalations repeated every 4 to 6 hours; in some patients, 1 inhalation every 4 hours may be sufficient. ( 2.1 ) Prime Levalbuterol tartrate HFA inhalation aerosol before using for the first time and when the inhaler has not been used for more than 3 days. To prime Levalbuterol tartrate HFA inhalation aerosol, release 4 sprays into the air away from the face. ( 2.2 ) At least once a week, wash the actuator with warm water and let it air-dry completely. ( 2.2 ) 2.1 Recommended Dosages The recommended dosage of Levalbuterol tartrate HFA inhalation aerosol for adults and children 4 years of age and older is 2 inhalations (90 mcg of levalbuterol free base) repeated every 4 to 6 hours; in some patients, 1 inhalation (45 mcg of levalbuterol free base) every 4 hours may be sufficient. More frequent administration or a larger number of inhalations is not routinely recommended. If a previously effective dosage regimen fails to provide the usual response, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids. 2.2 Administration Information For oral inhalation only Shake well before use. Avoid spraying in the eyes. Prime the inhaler before using for the first time and when the inhaler has not been used for more than 3 days by releasing 4 test sprays into the air, away from the face. To maintain proper use of Levalbuterol tartrate HFA inhalation aerosol, it is critical to wash the actuator with warm water and air-dry thoroughly at least once a week. The inhaler may cease to deliver levalbuterol tartrate if not properly cleaned and dried thoroughly. Keep the plastic actuator clean to prevent medication build-up and blockage. If the actuator becomes blocked with levalbuterol tartrate, wash the actuator to remove the blockage The canister is fitted with a dose indicator, which indicates how many inhalations remain. The dose indicator display will move after every tenth actuation. When nearing the end of the usable inhalations, the color behind the number in the dose indicator window changes to red. Discard the inhaler when the dose indicator display window shows zero, corresponding to the use of 200 actuations.
