Drug Catalog - Product Detail
LEVOBUNOLOL HCL SOL 0.005 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0505-05 | BAUSCH HEALTH | 5 | 0.5% | SOLUTION |
PACKAGE FILES

Generic Name
LEVOBUNOLOL HYDROCHLORIDE
Substance Name
LEVOBUNOLOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
ANDA074326
Description
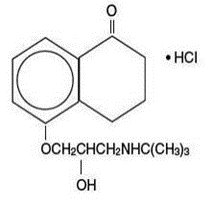
DESCRIPTION Levobunolol hydrochloride ophthalmic solution USP, 0.5% is a noncardioselective beta-adrenoceptor blocking agent for ophthalmic use. Levobunolol hydrochloride is represented by the following structural formula: Mol. Formula C 17 H 25 NO 3 •HCl Mol. Wt. 327.85 Chemical Name: (–)-5-[3-( tert -Butylamino)-2-hydroxypropoxy]-3,4-dihydro-1(2 H )- naphthalenone hydrochloride. Each mL of 0.5% contains: Active: levobunolol hydrochloride 0.5%; Inactives: polyvinyl alcohol 1.4%, sodium chloride, dibasic sodium phosphate, edetate disodium, sodium metabisulfite, monobasic potassium phosphate, and purified water. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (5.5 - 7.5); Preservative: benzalkonium chloride (0.004%). BETAGAN ® (levobunolol hydrochloride ophthalmic solution, USP) s terile
How Supplied
HOW SUPPLIED Levobunolol hydrochloride ophthalmic solution USP, 0.5% is supplied sterile in a plastic bottle with a controlled drop tip in the following sizes: NDC 24208-505-05 - 5 mL NDC 24208-505-10 - 10 mL NDC 24208-505-15 - 15 mL Storage: Store between 15°C to 25°C (59°F to 77°F). Protect from light. Replace cap immediately after use. KEEP OUT OF REACH OF CHILDREN. Distributed by: Bausch & Lomb Americas Inc. Bridgewater, NJ 08807 USA Manufactured by: Bausch & Lomb Incorporated Tampa, FL 33637 USA © 2022 Bausch & Lomb Incorporated or its affiliates Revised: October 2022 9117404 (Folded) 9117504 (Flat) Do not
Indications & Usage
INDICATIONS AND USAGE Levobunolol hydrochloride ophthalmic solution has been shown to be effective in lowering intraocular pressure and may be used in patients with chronic open-angle glaucoma or ocular hypertension.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended starting dose is one to two drops of levobunolol hydrochloride ophthalmic solution, 0.5% in the affected eye(s) once a day. In patients with more severe or uncontrolled glaucoma, levobunolol hydrochloride ophthalmic solution, 0.5% can be administered twice a day. As with any new medication, careful monitoring of patients is advised. Dosages above one drop of levobunolol hydrochloride ophthalmic solution, 0.5% twice a day are not generally more effective. If the patient's IOP is not at a satisfactory level on this regimen, concomitant therapy with other ophthalmic IOP-lowering agents can be instituted. Patients should not typically use two or more topical ophthalmic beta-adrenergic blocking agents simultaneously.
