Drug Catalog - Product Detail
LEVONORGESTREL/ETHINYL ESTRADIOL (JOLESSA) TB 0.15/0.03MG 3X91
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00555-9123-66 | TEVA PHARMACEUTICALS USA | 91 | 0.15-0.03MG | TABLET |
PACKAGE FILES




Generic Name
LEVONORGESTREL / ETHINYL ESTRADIOL
Substance Name
Product Type
HUMAN PRESCRIPTION DRUG
Route
Application Number
NDA021544
Description
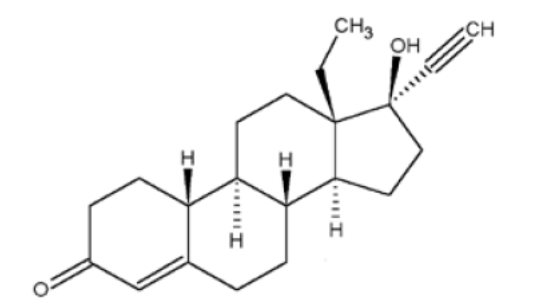
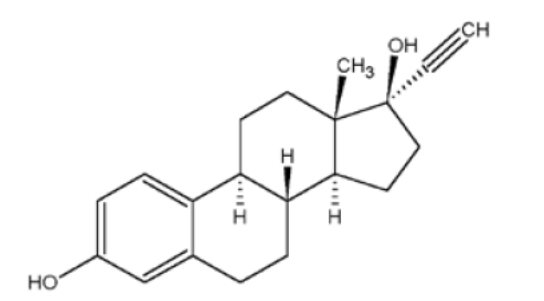
11 DESCRIPTION JOLESSA (levonorgestrel and ethinyl estradiol tablets) is an extended-cycle combination oral contraceptive consisting of 84 pink active tablets each containing 0.15 mg of levonorgestrel, a synthetic progestin and 0.03 mg of ethinyl estradiol, an estrogen, and 7 white inert tablets (without hormones). The structural formulas for the active components are: Levonorgestrel C 21 H 28 O 2 MW: 312.4 Levonorgestrel is chemically 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17α)-, (-)-. Ethinyl Estradiol C 20 H 24 O 2 MW: 296.4 Ethinyl Estradiol is 19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17α)-. Each pink active tablet contains the following inactive ingredients: anhydrous lactose NF, FD&C blue no. 1, FD&C red no. 40, hydroxypropyl methylcellulose USP, microcrystalline cellulose NF, polyethylene glycol NF, magnesium stearate NF, polysorbate 80 NF, and titanium dioxide USP. Each white inert tablet contains the following inactive ingredients: anhydrous lactose NF, hydroxypropyl methylcellulose USP, microcrystalline cellulose NF, and magnesium stearate NF. levonorgestrel ethinyl estradiol
How Supplied
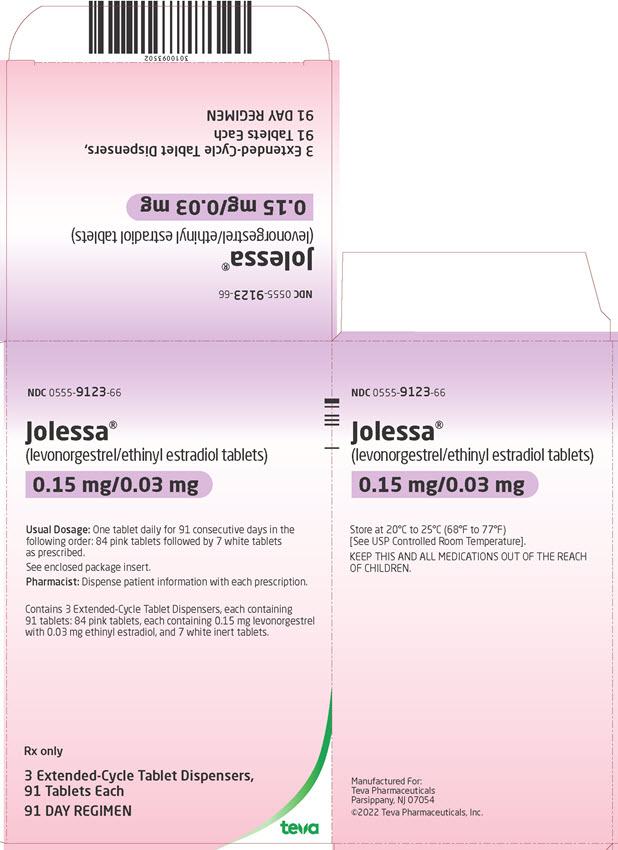
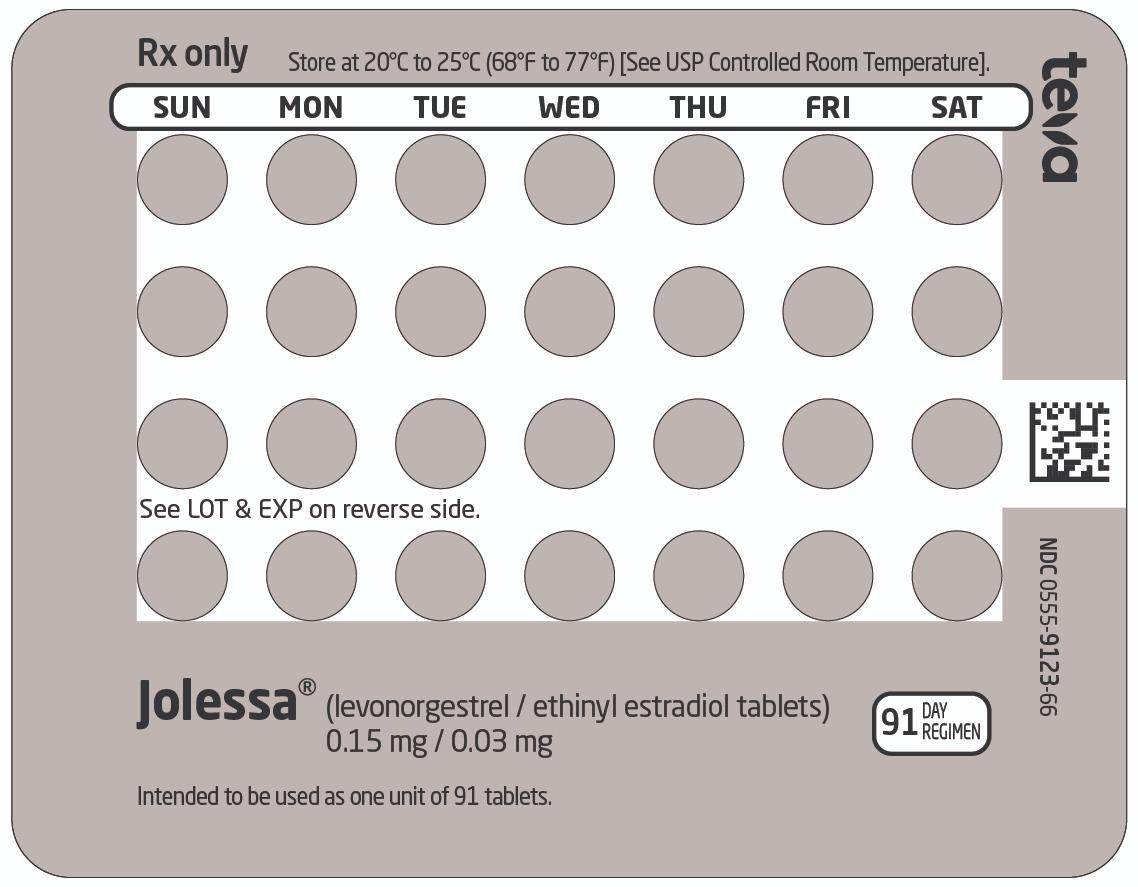
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied JOLESSA (levonorgestrel and ethinyl estradiol tablets) are available as round, film-coated, biconvex, unscored tablets with a debossed stylized b on one side, packaged in Extended-Cycle Tablet Dispensers, each containing a 13-week supply of tablets in the following order: 84 pink tablets, each containing 0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol: debossed with 992 on the other side 7 white inert tablets debossed with 208 on the other side Box of 3 Extended-Cycle Tablet Dispensers NDC 0555-9123-66 Storage and Handling Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [See USP Controlled Room Temperature]. Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE JOLESSA ® is indicated for use by females of reproductive potential to prevent pregnancy. JOLESSA is a combination of levonorgestrel, a progestin, and ethinyl estradiol, an estrogen, indicated for use by females of reproductive potential to prevent pregnancy. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Take one tablet daily by mouth at the same time every day for 91 days. ( 2.1 ) Take tablets in the order directed on the Extended-Cycle Tablet Dispenser. ( 2.2 ) 2.1 How to Start and Take JOLESSA JOLESSA is dispensed in an Extended-Cycle Tablet Dispenser [see How Supplied/Storage and Handling (16)]. JOLESSA should be started on a Sunday (see Table 1 ). For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration. Table 1: Instructions for Administration of JOLESSA Starting JOLESSA in females with no current use of hormonal contraception (Sunday Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product. Tablet Color: JOLESSA active tablets are pink (Day 1 to Day 84). JOLESSA inactive tablets are white (Day 85 to Day 91). Sunday Start: For each 91-day course, take in the following order: Take the first pink tablet (0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol) on the first Sunday after the onset of menstruation. If menstruation begins on a Sunday, take the tablet on that day. Due to the potential risk of becoming pregnant, use additional non-hormonal contraception (such as condoms or spermicide) for the first 7 days of treatment. Take subsequent pink tablets once daily at the same time each day for a total of 84 days. Take one white tablet (inert) daily for the following 7 days and at the same time of day that active tablets were taken. A scheduled period should occur during the 7 days that the white tablets are taken. Begin the next and all subsequent 91-day courses of JOLESSA without interruption on the same day of the week (Sunday) on which the patient began her first dose. Follow the same schedule as the initial 91-day course: a pink tablet once a day for 84 days, and a white tablet once a day for 7 days. If the patient does not immediately start her next pill pack, instruct her to protect herself from pregnancy by using a non-hormonal backup method of contraception until she has taken a pink tablet daily for 7 consecutive days. Switching from another contraceptive method to JOLESSA Start JOLESSA: Another oral contraceptive On the day when the new pack of the previous COC would have been started Transdermal patch On the day when the next application would have been scheduled. Vaginal ring On the day when the next insertion would have been scheduled. Injection On the day when the next injection would have been scheduled. Intrauterine contraceptive (IUD) On the day of removal. If the IUD is not removed on first day of the patient’s menstrual cycle, additional non-hormonal contraception (such as condoms or spermicide) is needed for the first seven days of the first 91-day course. Implant On the day of removal. Starting JOLESSA after Abortion or Miscarriage First-trimester After a first-trimester abortion or miscarriage, JOLESSA may be started immediately. An additional method of contraception is not needed if JOLESSA is started immediately. If JOLESSA is not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of her first 91-day course of JOLESSA. Second-trimester Do not start JOLESSA until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start JOLESSA following the instructions in Table 1 for Sunday start. Use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of the patient’s first 91-day course of JOLESSA [see Contraindications ( 4 ), Warnings and Precautions ( 5.1 )]. Starting JOLESSA after Childbirth Do not start JOLESSA until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with JOLESSA following the instructions in Table 1 for women not currently using hormonal contraception. JOLESSA is not recommended for use in lactating women [see Use in Specific Populations ( 8.2 )] . If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of JOLESSA [see Contraindications ( 4 ), Warnings and Precautions ( 5.1 ), Use in Specific Populations ( 8.1 and 8.2 )]. 2.2 Dosing JOLESSA Instruct patients to take one tablet by mouth at the same time every day. The dosing of JOLESSA is one pink pill containing levonorgestrel and ethinyl estradiol daily for 84 consecutive days, followed by one white pill (inactive pills without hormone) for 7 days. To achieve maximum contraceptive effectiveness, JOLESSA must be taken exactly as directed, in the order directed on the Tablet Dispenser, and at intervals not exceeding 24 hours. Start taking the first pink pill from a new Tablet Dispenser the very next day after taking the last white inactive pill in the Tablet Dispenser. The failure rate may increase when pills are missed or taken incorrectly. 2.3 Missed Doses Table 2: Instructions for Missed JOLESSA Tablets If one active tablet (pink) is missed in Days 1 through 84 Take the tablet as soon as possible. Take the next tablet at the regular time and continue taking one tablet a day until the 91-day course is finished. If two consecutive active tablets (pink) are missed in Days 1 through 84 Take 2 tablets on the day remembered and 2 tablets the next day. Then continue taking one tablet a day until the 91-day course is finished. Additional non-hormonal contraception (such as condoms or spermicide) should be used as backup if the patient has sex within 7 days after missing tablets. If three or more consecutive active tablets (pink) are missed in Days 1 through 84 Do not take the missed tablets. Continue taking one tablet a day until the 91-day course is finished. Additional non-hormonal contraception (such as condoms or spermicide) must be used as backup if the patient has sex within 7 days after missing tablets. If any of the seven white (inactive) tablets are missed Throw away the missed tablets. Continue taking the remaining tablets until the pack is finished. A backup birth control method is not needed. 2.4 Advice in Case of Gastrointestinal Disturbances In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3-4 hours after taking a pink tablet, handle this as a missed tablet.
