Drug Catalog - Product Detail
LIVALO TABS 2MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 66869-0204-90 | KOWA PHARMACEUTICALS AMERICA | 90 | 2MG | TABLET |
PACKAGE FILES




Generic Name
PITAVASTATIN CALCIUM
Substance Name
PITAVASTATIN CALCIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA022363
Description
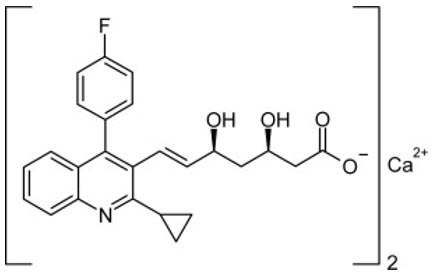
11 DESCRIPTION LIVALO (pitavastatin) tablets for oral use is an HMG-CoA reductase inhibitor. The chemical name for pitavastatin is (+)monocalcium bis {(3R, 5S, 6 E )-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate}. The structural formula is: The empirical formula for pitavastatin is C 50 H 46 CaF 2 N 2 O 8 and the molecular weight is 880.98. Pitavastatin is odorless and occurs as white to pale-yellow powder. It is freely soluble in pyridine, chloroform, dilute hydrochloric acid, and tetrahydrofuran, soluble in ethylene glycol, sparingly soluble in octanol, slightly soluble in methanol, very slightly soluble in water or ethanol, and practically insoluble in acetonitrile or diethyl ether. Pitavastatin is hygroscopic and slightly unstable in light. Each film-coated tablet of LIVALO contains 1 mg, 2 mg, or 4 mg of pitavastatin, which is equivalent to 1.045 mg, 2.09 mg, or 4.18 mg, respectively, of pitavastatin calcium and the following inactive ingredients: hypromellose, lactose monohydrate, low substituted hydroxypropylcellulose, magnesium aluminometasilicate, and magnesium stearate. The film coating contains the following inactive ingredients: colloidal anhydrous silica, hypromellose, titanium dioxide, and triethyl citrate. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING LIVALO tablets are supplied as follows: Tablet Strength Package Size Tablet Description NDC 1 mg Bottle of 90 Round white film-coated tablet debossed "KC" on one face and "1" on the reverse 66869-104-90 2 mg Bottle of 90 Round white film-coated tablet debossed "KC" on one face and "2" on the reverse 66869-204-90 4 mg Bottle of 90 Round white film-coated tablet debossed "KC" on one face and "4" on the reverse 66869-404-90 Store at room temperature between 15°C and 30°C (59° to 86° F) [see USP]. Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE LIVALO is indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in: Adults with primary hyperlipidemia. Adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH). LIVALO is a HMG-CoA reductase inhibitor (statin) indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in: ( 1 ) Adults with primary hyperlipidemia. Adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Take orally once daily with or without food at the same time each day. ( 2.1 ) For patients requiring a high-intensity statin or are unable to achieve their LDL-C goal receiving LIVALO 4 mg daily, prescribe alternative LDL-C-lowering treatment. ( 2.1 ) Assess LDL-C when clinically appropriate, as early as 4 weeks after initiation of LIVALO, and adjust the dosage if necessary. ( 2.1 ) Recommended dosage is 2 mg to 4 mg once daily. Maximum recommended dosage is 4 mg once daily. ( 2.2 ) Recommended starting dosage for patients with moderate and severe renal impairment and end-stage renal disease on hemodialysis is 1 mg once daily. Maximum recommended dosage is 2 mg once daily. ( 2.3 ) See full prescribing information for LIVALO dosage modifications due to drug interactions. ( 2.4 ) 2.1 Important Dosage and Administration Information Take LIVALO orally once daily with or without food at the same time each day. For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving LIVALO 4 mg daily, prescribe alternative LDL-C-lowering treatment. Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating LIVALO, and adjust the dosage if necessary. 2.2 Recommended Dosage for Adults and Pediatric Patients Aged 8 Years and Older The recommended dosage range of LIVALO is 2 mg to 4 mg daily. The maximum recommended dosage is LIVALO 4 mg once daily . 2.3 Recommended Dosage in Patients with Renal Impairment The recommended starting dosage for patients with moderate and severe renal impairment (estimated glomerular filtration rate 30 – 59 mL/minute/1.73 m 2 and 15 – 29 mL/minute/1.73 m 2 , respectively) and patients with end-stage renal disease receiving hemodialysis is LIVALO 1 mg once daily. The maximum recommended dose for these patients is LIVALO 2 mg once daily [see Use in Specific Populations (8.5) ] . There are no dosage adjustment recommendations for patients with mild renal impairment. 2.4 Dosage Modifications Due to Drug Interactions In patients taking erythromycin, do not exceed LIVALO 1 mg once daily [see Drug Interactions (7) ] . In patients taking rifampin, do not exceed LIVALO 2 mg once daily [see Drug Interactions (7) ] .
