Drug Catalog - Product Detail
LOVASTATIN USP 40MG TB 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61442-0143-10 | CARLSBAD TECHNOLOGIES | 1000 | 40MG | TABLET |
PACKAGE FILES



Generic Name
LOVASTATIN
Substance Name
LOVASTATIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075991
Description
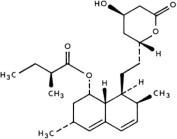
DESCRIPTION Lovastatin is a cholesterol lowering agent isolated from a strain of Aspergillus terreus. After oral ingestion, lovastatin, which is an inactive lactone, is hydrolyzed to the corresponding β-hydroxyacid form. This is a principal metabolite and an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and rate limiting step in the biosynthesis of cholesterol. Lovastatin is [1 S -[1α( R *), 3α, 7β, 8β(2 S *,4 S *),8aβ]]-1,2,3, 7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2 H -pyran-2-yl)ethyl]-1-naphthalenyl 2-methylbutanoate. The empirical formula of lovastatin is C 24 H 36 O 5 and its molecular weight is 404.55. Its structural formula is: Lovastatin is a white, nonhygroscopic crystalline powder that is insoluble in water and sparingly soluble in ethanol, methanol, and acetonitrile. Lovastatin tablets are supplied as 10 mg, 20 mg and 40 mg tablets for oral administration. In addition to the active ingredient lovastatin, each tablet contains the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer, pregelatinized starch, sodium starch glycolate, butylated hydroxyanisole and talc. Butylated hydroxyanisole (BHA) is added as a preservative. Structural Formula
How Supplied
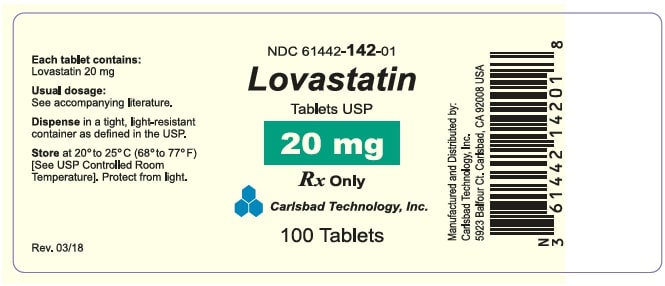
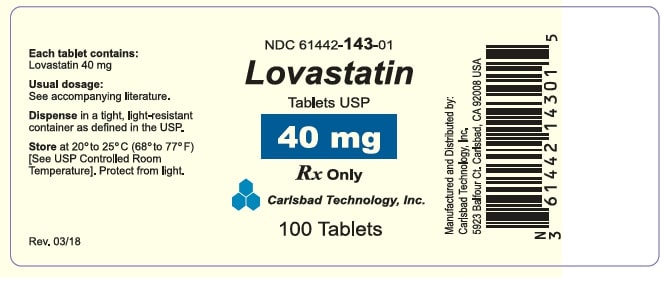
HOW SUPPLIED Lovastatin Tablets USP (white to off white round, unscored tablets) containing 10mg of lovastatin and engraved with “CTI 141" Bottle of 60........................................................................ (NDC 61442-141-60) Bottle of 90........................................................................ (NDC 61442-141-90) Bottle of 100...................................................................... (NDC 61442-141-01) Bottle of 500...................................................................... (NDC 61442-141-05) Bottle of 1,000................................................................... (NDC 61442-141-10) Lovastatin Tablets USP (white to off white round, unscored tablets) containing 20mg of lovastatin and engraved with “CTI 142" Bottle of 60........................................................................ (NDC 61442-142-60) Bottle of 90........................................................................ (NDC 61442-142-90) Bottle of 100...................................................................... (NDC 61442-142-01) Bottle of 500...................................................................... (NDC 61442-142-05) Bottle of 1,000................................................................... (NDC 61442-142-10) Lovastatin Tablets USP (white to off white round, unscored tablets) containing 40mg of lovastatin and engraved with “CTI 143" Bottle of 60........................................................................ (NDC 61442-143-60) Bottle of 90........................................................................ (NDC 61442-143-90) Bottle of 100...................................................................... (NDC 61442-143-01) Bottle of 500...................................................................... (NDC 61442-143-05) Bottle of 1,000................................................................... (NDC 61442-143-10) Storage Store at 20º to 25º C (68º to 77º F). [See USP Controlled Room Temperature.] Lovastatin Tablets must be protected from light and stored in a well-closed, light-resistant and child proof container. Manufactured and Distributed By: Carlsbad Technology, Inc. 5923 Balfour Court Carlsbad, CA 92008 Revised: 03/2024 CTI-13 Rev. N
Indications & Usage
Indications and Usage Therapy with lovastatin should be a component of multiple risk factor intervention in those individuals with dyslipidemia at risk for atherosclerotic vascular disease. Lovastatin should be used in addition to a diet restricted in saturated fat and cholesterol as part of a treatment strategy to lower total-C and LDL-C to target levels when the response to diet and other nonpharmacological measures alone has been inadequate to reduce risk. Primary Prevention of Coronary Heart Disease In individuals without symptomatic cardiovascular disease, average to moderately elevated total-C and LDL-C, and below average HDL-C, lovastatin is indicated to reduce the risk of: Myocardial infarction Unstable angina Coronary revascularization procedures (See CLINICAL PHARMACOLOGY , Clinical Studies .) Coronary Heart Disease Lovastatin is indicated to slow the progression of coronary atherosclerosis in patients with coronary heart disease as part of a treatment strategy to lower total-C and LDL-C to target levels. Hypercholesterolemia Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for artherosclerotic vascular disease due to hypercholesterolemia. Lovastatin is indicated as an adjunct to diet for the reduction of elevated total-C and LDL-C levels in patients with primary hypercholesterolemia (Types IIa and IIb 2 ), when the response to diet restricted in saturated fat and cholesterol and to other nonpharmacological measures alone has been inadequate. 2 Classification of Hyperlipoproteinemias Lipid Elevaions Type Lipoproteins elevated major minor I chylonicrons TG ↑→C IIa LDL C -- IIb LDL, VLDL C TG III (rare) IDL C/TG -- IV VLDL TG ↑→C V (rare) chylomicrons, VLDL TG ↑→C IDL = intermediate-densit lipoprotein Adolescent Patients with Heterozygous Familial Hypercholesterolemia Lovastatin is indicated as an adjunct to diet to reduce total-C, LDL-C and apolipoprotein B levels in adolescent boys and girls who are at least one year post-menarche, 10 to 17 years of age, with heFH if after an adequate trial of diet therapy the following findings are present: LDL-C remains >189 mg/dL or LDL-C remains >160 mg/dL and: there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the adolescent patient General Recommendations Prior to initiating therapy with lovastatin, secondary causes for hypercholesterolemia (e.g., poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, alcoholism) should be excluded, and a lipid profile performed to measure total-C, HDL-C, and TG. For patients with TG less than 400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation: LDL-C = total-C - [0.2 × (TG) + HDL-C] For TG levels >400 mg/dL (>4.5 mmol/L), this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation. In hypertriglyceridemic patients, LDL-C may be low or normal despite elevated total-C. In such cases, lovastatin is not indicated. The National Cholesterol Education Program (NCEP) Treatment Guidelines are summarized below: NCEP Treatment Guidelines: LDL-C Goals and Cutpoints for Therapeutic Lifestyle Changes and Drug Therapy in Different Risk Categories Risk Category LDL Goal (mg/dL) LDL Level at Which to Initiate Therapeutic Lifestyle Changes (mg/dL) LDL Level at Which to Consider Drug Therapy (mg/dL) CHD† or CHD risk equivalents (10-year risk >20%) <100 ≥100 ≥130 (100 to 129: drug optional) †† 2+ Risk factors (10 year risk ≤20%) <130 ≥130 10-year risk 10 to 20%: ≥130 10-year risk <10%: ≥160 0 to 1 Risk factor ††† <160 ≥160 ≥190 (160 to 189: LDL-lowering drug optional) † CHD, coronary heart disease †† Some authorities recommend use of LDL-lowering drugs in this category if an LDL-C level of <100 mg/dL cannot be achieved by therapeutic lifestyle changes. Others prefer use of drugs that primarily modify triglycerides and HDL-C, e.g., nicotinic acid or fibrate. Clinical judgment also may call for deferring drug therapy in this subcategory. ††† Almost all people with 0 to 1 risk factor have a 10-year risk <10%; thus, 10-year risk assessment in people with 0 to 1 risk factor is not necessary. After the LDL-C goal has been achieved, if the TG is still ≥200 mg/dL, non-HDL-C (total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category. At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C is ≥130 mg/dL (see NCEP Guidelines above). Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the total-C be used to monitor therapy. Although lovastatin may be useful to reduce elevated LDL-C levels in patients with combined hypercholesterolemia and hypertriglyceridemia where hypercholesterolemia is the major abnormality (Type IIb hyperlipoproteinemia), it has not been studied in conditions where the major abnormality is elevation of chylomicrons, VLDL or IDL (i.e., hyperlipoproteinemia types I, III, IV, or V). *** The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below: Category Total-C (mg/dL) LDL-C (mg/dL) Acceptable <170 <110 Borderline 170 to 199 110 to 129 High ≥200 ≥130 Children treated with lovastatin in adolescence should be re-evaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult goals for LDL-C.
Dosage and Administration
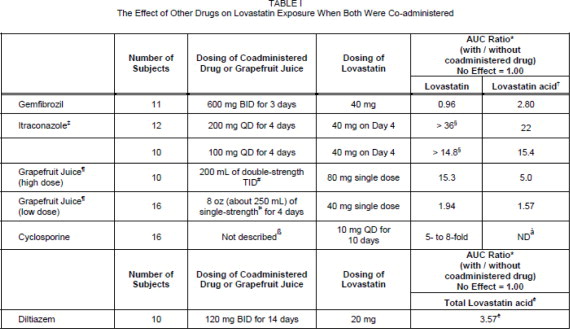
Dosage and Administration The patient should be placed on a standard cholesterol-lowering diet before receiving lovastatin and should continue on this diet during treatment with lovastatin (see NCEP Treatment Guidelines for details on dietary therapy). Lovastatin should be given with meals. Adult Patients The usual recommended starting dose is 20 mg once a day given with the evening meal. The recommended dosing range is 10 to 80 mg/day in single or two divided doses; the maximum recommended dose is 80 mg/day. Doses should be individualized according to the recommended goal of therapy (see NCEP Guidelines and CLINICAL PHARMACOLOGY ). Patients requiring reductions in LDL-C of 20% or more to achieve their goal (see INDICATIONS AND USAGE ) should be started on 20 mg/day of lovastatin. A starting dose of 10 mg may be considered for patients requiring smaller reductions. Adjustments should be made at intervals of 4 weeks or more. Cholesterol levels should be monitored periodically and consideration should be given to reducing the dosage of lovastatin if cholesterol levels fall significantly below the targeted range. Dosage in Patients taking Danazol, Diltiazem, Dronedarone, or Verapamil In patients taking danazol, diltiazem, dronedarone or verapamil concomitantly with lovastatin, therapy should begin with 10 mg of lovastatin and should not exceed 20 mg / day (see CLINICAL PHARMACOLOGY , Pharmacokinetics , WARNINGS , Myopathy/Rhabdomyolysis , PRECAUTIONS , Drug Interactions, Other Drug Interactions ). Dosage in Patients taking Amiodarone In patients taking amiodarone concomitantly with lovastatin, the dose should not exceed 40 mg/day (see WARNINGS , Myopathy/Rhabdomyolysis and PRECAUTIONS , Drug Interactions , Other drug interactions ). Adolescent Patients (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia The recommended dosing range is 10 to 40 mg/day; the maximum recommended dose is 40 mg/day. Doses should be individualized according to the recommended goal of therapy (see NCEP Pediatric Panel Guidelines ††, CLINICAL PHARMACOLOGY , and INDICATIONS AND USAGE ). Patients requiring reductions in LDL-C of 20% or more to achieve their goal should be started on 20 mg/day of lovastatin. A starting dose of 10 mg may be considered for patients requiring smaller reductions. Adjustments should be made at intervals of 4 weeks or more. Concomitant Lipid-Lowering Therapy Lovastatin is effective alone or when used concomitantly with bile-acid sequestrants (see WARNINGS , Myopathy/Rhabdomyolysis and PRECAUTIONS , Drug Interactions ). Dosage in Patients with Renal Insufficiency In patients with severe renal insufficiency (creatinine clearance <30 mL/min), dosage increases above 20 mg/day should be carefully considered and, if deemed necessary, implemented cautiously (see CLINICAL PHARMACOLOGY and WARNINGS , Myopathy/Rhabdomyolysis ). 4 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics . 89(3): 495-501, 1992.
