Drug Catalog - Product Detail
MELOXICAM TB 7.5MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0501-03 | LUPIN PHARMACEUTICALS | 1000 | 7.5MG | TABLET |
PACKAGE FILES
Generic Name
MELOXICAM
Substance Name
MELOXICAM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077944
Description
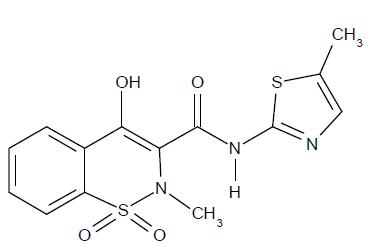
11 DESCRIPTION Meloxicam, is a nonsteroidal anti-inflammatory drug (NSAID). Each light yellow meloxicam tablet USP contains 7.5 mg or 15 mg meloxicam for oral administration. Meloxicam is chemically designated as 4-hydroxy-2-methyl- N -(5-methyl-2-thiazolyl)-2 H -1,2-benzothiazine-3-carboxamide-1,1-dioxide. The molecular weight is 351.4. Its empirical formula is C 14 H 13 N 3 O 4 S 2 and it has the following structural formula: Meloxicam is a pastel yellow solid, practically insoluble in water, with higher solubility observed in strong acids and bases. It is very slightly soluble in methanol. Meloxicam has an apparent partition coefficient (log P) app = 0.1 in n -octanol/buffer pH 7.4. Meloxicam has pKa values of 1.1 and 4.2. Meloxicam tablets USP are available as tablets for oral administration containing 7.5 mg or 15 mg meloxicam. The inactive ingredients in meloxicam tablets USP include colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium citrate dihydrate. image 01
How Supplied
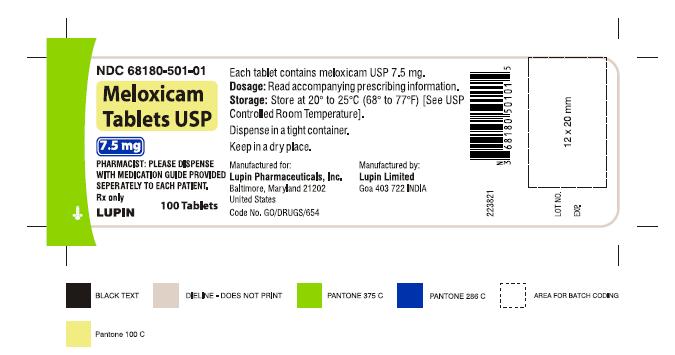
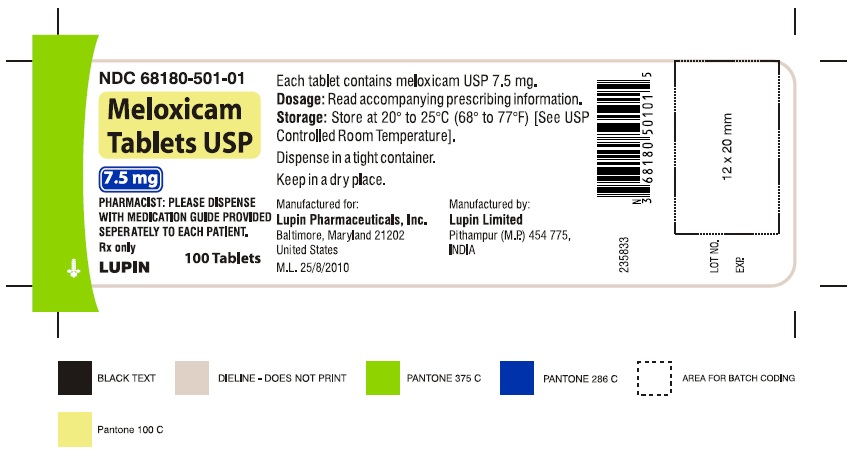
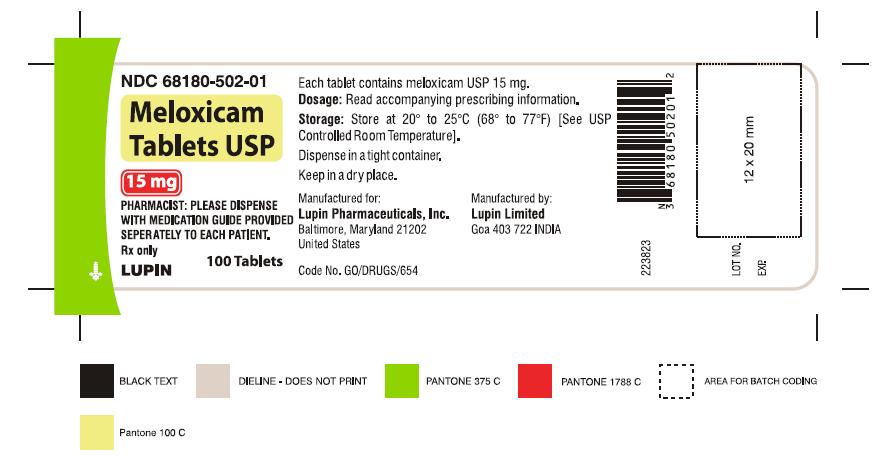
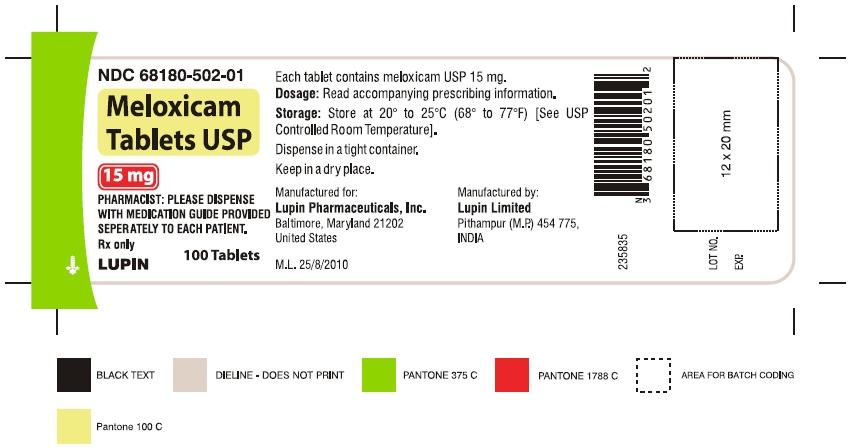
16 HOW SUPPLIED/STORAGE AND HANDLING Meloxicam Tablets USP are available as light yellow coloured, round, biconvex tablets, plain on one side and debossed with '7.5' on other side containing meloxicam 7.5 mg or as light yellow coloured, oval shaped, biconvex tablets, plain on one side and debossed with '15' on other side containing meloxicam 15 mg. Meloxicam Tablets USP, 7.5 mg are available as follows: Bottles of 100 NDC 68180-501-01 Bottles of 1000 NDC 68180-501-03 Meloxicam Tablets USP, 15 mg are available as follows: Bottles of 100 NDC 68180-502-01 Bottles of 1000 NDC 68180-502-03 Storage Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Keep meloxicam tablets USP in a dry place. Dispense tablets in a tight container. Keep this and all medications out of the reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Meloxicam tablet USP is a non-steroidal anti-inflammatory drug indicated for: Osteoarthritis (OA) ( 1.1 ) Rheumatoid Arthritis (RA) ( 1.2 ) Juvenile Rheumatoid Arthritis (JRA) in patients who weigh ≥60 kg ( 1.3 ) 1.1 Osteoarthritis (OA Meloxicam tablets USP are indicated for relief of the signs and symptoms of osteoarthritis [see Clinical Studies ( 14.1 )] . 1.2 Rheumatoid Arthritis (RA) Meloxicam tablets USP are indicated for relief of the signs and symptoms of rheumatoid arthritis [see Clinical Studies ( 14.1 )] . 1.3 Juvenile Rheumatoid Arthritis (JRA) Pauciarticular and Polyarticular Course Meloxicam tablets USP are indicated for relief of the signs and symptoms of pauciarticular or polyarticular course Juvenile Rheumatoid Arthritis in patients who weigh ≥60 kg [see Dosage and Administration ( 2.4 ) and Clinical Studies ( 14.2 )] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals ( 2.1 ) OA ( 2.2 ) and RA (2.3): Starting dose: 7.5 mg once daily Dose may be increased to 15 mg once daily JRA ( 2.4 ): 7.5 mg once daily in children ≥60 kg Meloxicam Tablets are not interchangeable with approved formulations of oral meloxicam even if the total milligram strength is the same ( 2.6 ) 2.1 General Dosing Instructions Carefully consider the potential benefits and risks of meloxicam tablets and other treatment options before deciding to use meloxicam tablets. Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions ( 5 )] . After observing the response to initial therapy with meloxicam tablets, adjust the dose to suit an individual patient's needs. In adults, the maximum recommended daily oral dose of meloxicam tablets is 15 mg regardless of formulation. In patients with hemodialysis, a maximum daily dosage of 7.5 mg is recommended [see Use in Specific Populations ( 8.7 ) and Clinical Pharmacology ( 12.3 )] . Meloxicam tablets may be taken without regard to timing of meals. 2.2 Osteoarthritis For the relief of the signs and symptoms of osteoarthritis the recommended starting and maintenance oral dose of meloxicam tablets is 7.5 mg once daily. Some patients may receive additional benefit by increasing the dose to 15 mg once daily. 2.3 Rheumatoid Arthritis For the relief of the signs and symptoms of rheumatoid arthritis, the recommended starting and maintenance oral dose of meloxicam tablets is 7.5 mg once daily. Some patients may receive additional benefit by increasing the dose to 15 mg once daily. 2.4 Juvenile Rheumatoid Arthritis (JRA) Pauciarticular and Polyarticular Course For the treatment of juvenile rheumatoid arthritis, the recommended oral dose of meloxicam is 7.5 mg once daily in children who weigh ≥60 kg. There was no additional benefit demonstrated by increasing the dose above 7.5 mg in clinical trials. Meloxicam tablets should not be used in children who weigh <60 kg. 2.5 Renal Impairment The use of meloxicam in subjects with severe renal impairment is not recommended. In patients on hemodialysis, the maximum dosage of meloxicam is 7.5 mg per day [see Clinical Pharmacology ( 12.3 )]. 2.6 Non-Interchangeability with Other Formulations of Meloxicam Meloxicam Tablets have not shown equivalent systemic exposure to other approved formulations of oral meloxicam. Therefore, Meloxicam Tablets are not interchangeable with other formulations of oral meloxicam product even if the total milligram strength is the same. Do not substitute similar dose strengths of Meloxicam Tablets with other formulations of oral meloxicam product.
