Drug Catalog - Product Detail
MEMANTINE HCI TAB 5MG AMN 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 53746-0173-60 | AMNEAL PHARMACEUTICALS | 60 | 5MG | TABLET |
PACKAGE FILES


Generic Name
MEMANTINE
Substance Name
MEMANTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090041
Description
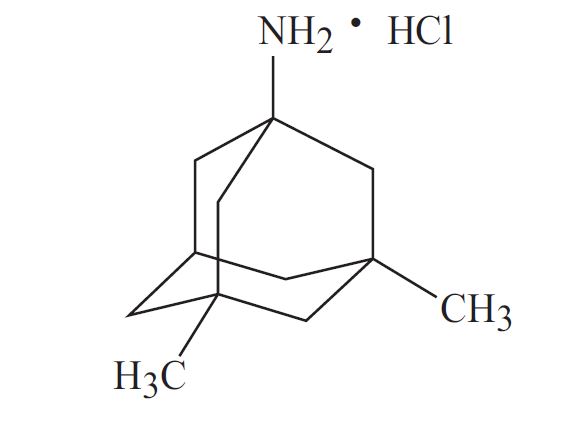
11 DESCRIPTION Memantine hydrochloride, USP is an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride, USP is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural formula: The molecular formula is C 12 H 21 N•HCl and the molecular weight is 215.76. Memantine hydrochloride, USP occurs as a fine white to off-white powder and is soluble in water. Memantine hydrochloride, USP is available for oral administration as oblong, film-coated tablets containing either 5 mg or 10 mg of memantine hydrochloride, USP. The tablets also contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose and talc. In addition the following inactive ingredients are also present as components of the film coat: D&C Yellow #10, FD&C Blue #2, FD&C Yellow #6, macrogol, polyvinyl alcohol, talc, and titanium dioxide (5 mg tablets), and iron oxide black, iron oxide yellow, macrogol, polyvinyl alcohol, talc and titanium dioxide (10 mg tablets). 1
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Memantine Hydrochloride Tablets USP, 5 mg, are supplied as orange colored, oblong, biconvex, film-coated tablets debossed “IP 173” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 53746-173-30 Bottles of 60: NDC 53746-173-60 Bottles of 1000: NDC 53746-173-10 Memantine Hydrochloride Tablets USP, 10 mg, are supplied as gray colored, oblong, biconvex, film-coated tablets debossed “IP 174” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 53746-169-30 Bottles of 60: NDC 53746-169-60 Bottles of 1000: NDC 53746-169-10 Store Memantine Hydrochloride Tablets, USP at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Memantine hydrochloride tablets are indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. Memantine hydrochloride tablets are an N-methyl-D-aspartate (NMDA) receptor antagonist indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The recommended starting dose of memantine hydrochloride tablets is 5 mg once daily. The dose should be increased in 5 mg increments to 10 mg/day (5 mg twice daily), 15 mg/day (5 mg and 10 mg as separate doses), and 20 mg/day (10 mg twice daily). The minimum recommended interval between dose increases is one week. The dosage shown to be effective in controlled clinical trials is 20 mg/day. Memantine hydrochloride tablets can be taken with or without food. If a patient misses a single dose of memantine hydrochloride tablets, that patient should not double up on the next dose. The next dose should be taken as scheduled. If a patient fails to take memantine hydrochloride tablets for several days, dosing may need to be resumed at lower doses and retitrated as described above. Specific Populations Renal Impairment A target dose of 5 mg twice daily is recommended in patients with severe renal impairment (creatinine clearance of 5 to 29 mL/min based on the Cockcroft-Gault equation). Hepatic Impairment Memantine hydrochloride tablets should be administered with caution to patients with severe hepatic impairment [see Clinical Pharmacology (12.3) ] . May be taken with or without food. (2) Initial dose is 5 mg once daily. Increase dose in 5 mg increments to a maintenance dose of 10 mg twice daily. A minimum of 1 week of treatment with the previous dose should be observed before increasing the dose. (2) Severe renal impairment: recommended dose is 5 mg twice daily. (2)
