Drug Catalog - Product Detail
MEMANTINE HCL ER 28MG CP 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0785-03 | AMNEAL PHARMACEUTICALS | 30 | 28MG | CAPSULE |
PACKAGE FILES
Generic Name
MEMANTINE HYDROCHLORIDE
Substance Name
MEMANTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205825
Description
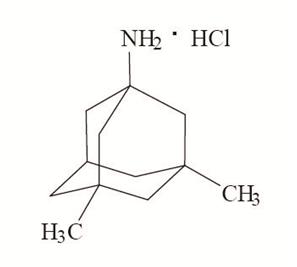
11 DESCRIPTION Memantine hydrochloride extended-release capsules are an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride, USP is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural formula: The molecular formula is C 12 H 21 N•HCl and the molecular weight is 215.76. Memantine hydrochloride, USP occurs as a fine white to off-white powder and is soluble in water. Memantine hydrochloride extended-release capsules are supplied for oral administration as 7 mg, 14 mg, 21 mg, and 28 mg capsules. Each capsule contains extended-release beads with the labeled amount of memantine hydrochloride, USP and the following inactive ingredients: colloidal silicon dioxide, ethylcellulose, hypromellose, sugar spheres (sucrose, corn starch), talc, and triethyl citrate in hard gelatin capsules. Each capsule shell contains the following inactive ingredients: D&C Yellow No. 10, gelatin, iron oxide yellow and titanium dioxide. The 14 mg, 21 mg and 28 mg capsule shells also contain FD&C Blue No. 1. The 7 mg, 14 mg and 21 mg imprinting ink contains the following inactive ingredients: D&C Yellow No. 10, FD&C Blue No. 1, FD&C Blue No. 2, FD&C Red No. 40, iron oxide black, N-butyl alcohol, propylene glycol and shellac glaze. The 28 mg imprinting ink contains the following inactive ingredients: ammonium hydroxide, isopropyl alcohol, N-butyl alcohol, propylene glycol, simethicone, shellac glaze and titanium dioxide. Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Memantine hydrochloride extended-release capsules, 7 mg, are supplied as a two-piece hard gelatin capsule with standard yellow opaque cap and standard yellow opaque body filled with white to off-white pellets. Imprinted in black ink “AN” on the cap and imprinted in black ink “782” on the body. They are available as follows: Bottles of 30: NDC 65162-782-03 Bottles of 90: NDC 65162-782-09 Bottles of 500: NDC 65162-782-50 Memantine hydrochloride extended-release capsules, 14 mg, are supplied as a two-piece hard gelatin capsule with standard yellow opaque cap and dark green opaque body filled with white to off-white pellets. Imprinted in black ink “AN” on the cap and imprinted in black ink “783”on the body. They are available as follows: Bottles of 30: NDC 65162-783-03 Bottles of 90: NDC 65162-783-09 Bottles of 500: NDC 65162-783-50 Memantine hydrochloride extended-release capsules, 21 mg, are supplied as a two-piece hard gelatin capsule with white opaque cap and dark green opaque body filled with white to off-white pellets. Imprinted in black ink “AN” on the cap and imprinted in black ink “784” on the body. They are available as follows: Bottles of 30: NDC 65162-784-03 Bottles of 90: NDC 65162-784-09 Bottles of 500: NDC 65162-784-50 Memantine hydrochloride extended-release capsules, 28 mg, are supplied as a two-piece hard gelatin capsule with dark green opaque cap and dark green opaque body filled with white to off-white pellets. Imprinted in white ink “AN” on the cap and imprinted in white ink “785” on the body. They are available as follows: Bottles of 30: NDC 65162-785-03 Bottles of 90: NDC 65162-785-09 Bottles of 500: NDC 65162-785-50 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
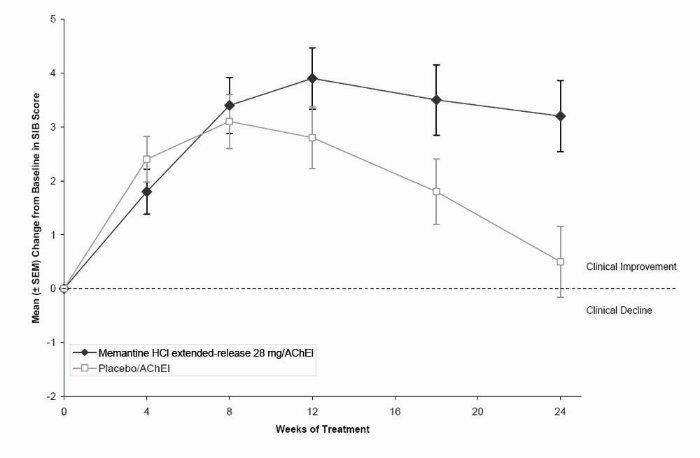
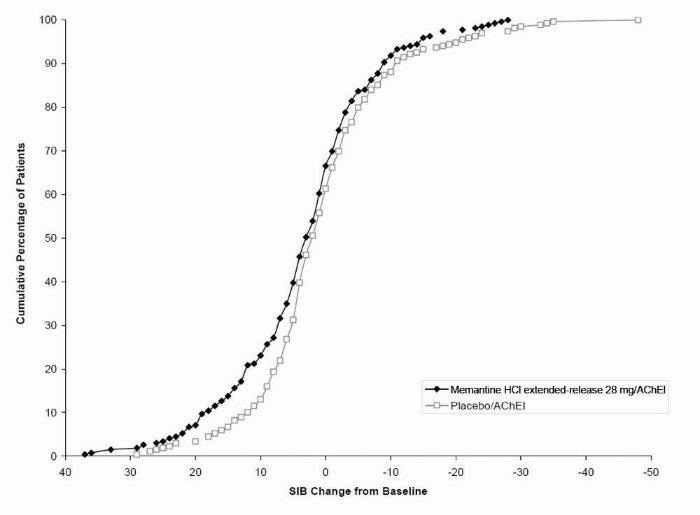
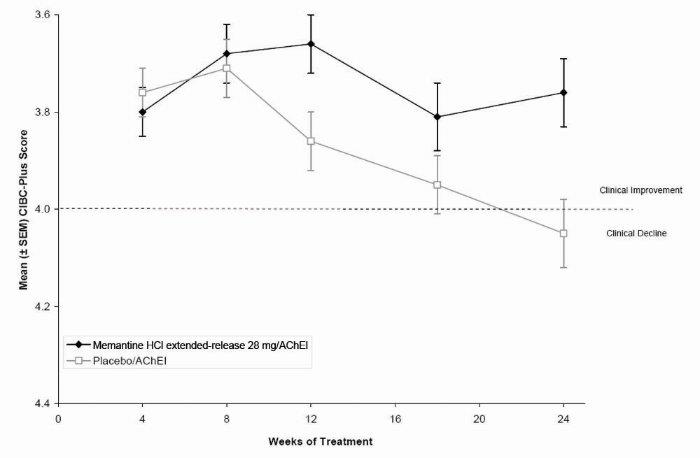
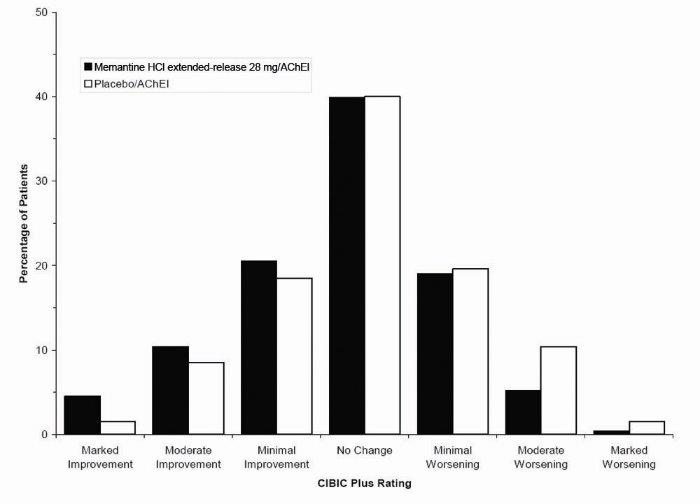
1 INDICATIONS AND USAGE Memantine hydrochloride extended-release capsules are indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. Memantine hydrochloride extended-release capsules are a N-methyl-D-aspartate (NMDA) receptor antagonist indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The recommended starting dose of memantine hydrochloride extended-release capsules is 7 mg once daily; the dose should be increased in 7 mg increments to the recommended maintenance dose of 28 mg once daily; the minimum recommended interval between dose increases is one week. ( 2.1 ) Patients with severe renal impairment: the recommended maintenance dose of memantine hydrochloride extended-release capsules is 14 mg once daily. ( 2.3 ) 2.1 Recommended Dosing The dosage of memantine hydrochloride extended-release capsules shown to be effective in a controlled clinical trial is 28 mg once daily. The recommended starting dose of memantine hydrochloride extended-release capsules is 7 mg once daily. The dose should be increased in 7 mg increments to the recommended maintenance dose of 28 mg once daily. The minimum recommended interval between dose increases is one week. The dose should only be increased if the previous dose has been well tolerated. The maximum recommended dose is 28 mg once daily. Memantine hydrochloride extended-release capsules can be taken with or without food. Memantine hydrochloride extended-release capsules can be taken intact or may be opened, sprinkled on applesauce, and thereby swallowed. The entire contents of each memantine hydrochloride extended-release capsule should be consumed; the dose should not be divided. Except when opened and sprinkled on applesauce, as described above, memantine hydrochloride extended-release capsules should be swallowed whole. Memantine hydrochloride extended-release capsules should not be divided, chewed, or crushed. If a patient misses a single dose of memantine hydrochloride extended-release capsules, that patient should not double up on the next dose. The next dose should be taken as scheduled. If a patient fails to take memantine hydrochloride extended-release capsules for several days, dosing may need to be resumed at lower doses and retitrated as described above. 2.2 Switching from Memantine hydrochloride to Memantine hydrochloride Extended-release Capsules Patients treated with memantine hydrochloride may be switched to memantine hydrochloride extended-release capsules as follows: It is recommended that a patient who is on a regimen of 10 mg twice daily of memantine hydrochloride be switched to memantine hydrochloride extended-release 28 mg once daily capsules the day following the last dose of 10 mg memantine hydrochloride. There is no study addressing the comparative efficacy of these 2 regimens. In a patient with severe renal impairment, it is recommended that a patient who is on a regimen of 5 mg twice daily of memantine hydrochloride be switched to memantine hydrochloride extended-release 14 mg once daily capsules the day following the last dose of 5 mg memantine hydrochloride. 2.3 Dosing in Patients with Renal Impairment In patients with severe renal impairment (creatinine clearance of 5 to 29 mL/min, based on the Cockcroft-Gault equation), the recommended maintenance dose (and maximum recommended dose) is 14 mg/day [see Clinical Pharmacology (12.3) ] .
