Drug Catalog - Product Detail
METFORMIN HCL TB 1000MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67877-0221-10 | ASCEND LABORATORIES | 1000 | 1000MG | TABLET |
PACKAGE FILES







Generic Name
METFORMIN HYDROCHLORIDE
Substance Name
METFORMIN HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091184
Description
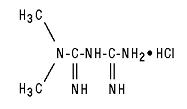
11 DESCRIPTION Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin hydrochloride is N,N -dimethylimidodicarbonimidic diamide hydrochloride. The structural formula is as shown below: Metformin hydrochloride USP is a white to off-white crystalline compound with a molecular formula of C 4 H 11 N 5 • HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. Metformin hydrochloride tablets USP contain 500 mg, 850 mg, or 1000 mg of metformin hydrochloride USP. Each tablet contains the inactive ingredients corn starch, povidone and magnesium stearate. In addition, the coating for each tablet contains hypromellose, talc, titanium dioxide, polyethylene glycol, and propylene glycol. Metformin hydrochloride extended-release tablets, USP contains 500 mg or 750 mg of metformin hydrochloride as the active ingredient. Metformin hydrochloride extended-release tablets USP, 500 mg and 750 mg contain the inactive ingredients sodium carboxymethyl cellulose, hypromellose, microcrystalline cellulose, magnesium stearate and copovidone. FDA approved dissolution test specifications differ from USP. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 HOW SUPPLIED Table 13: Metformin Hydrochloride Tablets, USP and Metformin Hydrochloride Extended-Release Tablets, USP Available Strengths, Units, and Appearance Metformin Hydrochloride Tablets, USP 500 mg Bottles of 100 NDC 67877-217-01 Metformin hydrochloride tablets, USP 500 mg are white to off-white, round shape, biconvex coated tablets debossed with "227" on one side and plain on the other side. Bottles of 500 NDC 67877-217-05 Bottles of 1000 NDC 67877-217-10 Blister Pack of 100 (10x10) Unit Dose Tablets NDC 67877-217-38 10's Blister Pack NDC 67877-217-33 850 mg Bottles of 100 NDC 67877-218-01 Metformin hydrochloride tablets, USP 850 mg are white to off-white, round shape, biconvex coated tablets debossed with "228" on one side and plain on the other side. Bottles of 500 NDC 67877-218-05 Bottles of 1000 NDC 67877-218-10 Blister Pack of 100 (10x10) Unit Dose Tablets NDC 67877-218-38 10's Blister Pack NDC 67877-218-33 1000 mg Bottles of 100 NDC 67877-221-01 Metformin hydrochloride tablets, USP 1000 mg are white to off-white, oval, capsule shaped, biconvex coated tablets debossed with “229/229” on one side and a bisect line on both sides. Bottles of 500 NDC 67877-221-05 Bottles of 1000 NDC 67877-221-10 Blister Pack of 100 (10x10) Unit Dose Tablets NDC 67877-221-38 10's Blister Pack NDC 67877-221-33 Metformin Hydrochloride Extended-Release Tablets, USP 500 mg Bottles of 90 NDC 67877-413-90 Metformin hydrochloride extended-release tablets USP, 500 mg are white to off-white, oval shaped, biconvex tablets with "MX" debossed on one side and "500" on other side. Bottles of 100 NDC 67877-413-01 Bottles of 500 NDC 67877-413-05 Bottles of 1000 NDC 67877-413-10 Carton of 100 (10 x 10 blisters) Unit Dose Tablets NDC 67877-413-33 750 mg Bottles of 90 NDC 67877-414-90 Metformin hydrochloride extended-release tablets USP, 750 mg are white to off-white, capsule shaped, tablets with "MX" debossed on one side and "750" on other side. Bottles of 100 NDC 67877-414-01 Bottles of 500 NDC 67877-414-05 Bottles of 1000 NDC 67877-414-10 Carton of 100 (10 x 10 blisters) Unit Dose Tablets NDC 67877-414-33 16.2 STORAGE Store at 20°–25°C (68°–77°F); excursions permitted to 15°–30°C (59°–86°F). [See USP Controlled Room Temperature.] Dispense in light-resistant containers.
Indications & Usage
1 INDICATIONS & USAGE Metformin hydrochloride tablets is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus. Metformin hydrochloride extended-release tablets is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Metformin is a biguanide indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus. (1) Metformin is a biguanide indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Dosage and Administration
2 DOSAGE & ADMINISTRATION Adult Dosage for Metformin Hydrochloride Tablets: Starting dose: 500 mg orally twice a day or 850 mg once a day, with meals (2.1) Increase the dose in increments of 500 mg weekly or 850 mg every 2 weeks, up to a maximum dose of 2550 mg per day, given in divided doses (2.1) Doses above 2000 mg may be better tolerated given 3 times a day with meals (2.1) Adult Dosage for Metformin Hydrochloride Extented-Release Tablets: Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew (2.1) Starting dose: 500 mg orally once daily with the evening meal (2.1) Increase the dose in increments of 500 mg weekly, up to a maximum of 2000 mg once daily with the evening meal (2.1) Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily (2.1) Pediatric Dosage for Metformin Hydrochloride Tablets: Starting dose: 500 mg orally twice a day, with meals (2.2) Increase dosage in increments of 500 mg weekly up to a maximum of 2000 mg per day, given in divided doses twice daily (2.2) Renal Impairment: • Prior to initiation, assess renal function with estimated glomerular filtration rate (eGFR) (2.3) Do not use in patients with eGFR below 30 mL/minute/1.73 m 2 (2.3) Initiation is not recommended in patients with eGFR between 30-45 mL/minute/1.73 m 2 (2.3) Asses risk/benefit of continuing if eGFR falls below 45 mL/minute/1.73 m 2 (2.3) Discontinue if eGFR falls below 30 mL/minute/1.73 m 2 (2.3) Discontinuation for Iodinated Contrast Imaging Procedures: • Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures (2.4) 2.1 Adult Dosage Metformin Hydrochloride Tablets The recommended starting dose of metformin hydrochloride tablets is 500 mg orally twice a day or 850 mg once a day, given with meals. Increase the dose in increments of 500 mg weekly or 850 mg every 2 weeks on the basis of glycemic control and tolerability, up to a maximum dose of 2550 mg per day, given in divided doses. Doses above 2000 mg may be better tolerated given 3 times a day with meals. Metformin Hydrochloride Extended-Release Tablets Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew. The recommended starting dose of metformin hydrochloride extended-release tablets is 500 mg orally once daily with the evening meal. Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg once daily with the evening meal. If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2550 mg administered in divided daily doses, as described above. Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily. 2.2 Pediatric Dosage for metformin hydrochloride tablets The recommended starting dose of metformin hydrochloride tablets for pediatric patients 10 years of age and older is 500 mg orally twice a day, given with meals. Increase dosage in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg per day, given in divided doses twice daily. 2.3 Recommendations for Use in Renal Impairment Assess renal function prior to initiation of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets and periodically thereafter. Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets is contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m 2 . Initiation of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets in patients with an eGFR between 30 – 45 mL/minute/1.73 m 2 is not recommended. In patients taking metformin hydrochloride tablets and metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m 2 , assess the benefit risk of continuing therapy. Discontinue metformin hydrochloride tablets and metformin hydrochloride extended-release tablets if the patient’s eGFR later falls below 30 mL/minute/1.73 m 2 [see Warnings and Precautions ( 5.1 ) ]. 2.4 Discontinuation for Iodinated Contrast Imaging Procedures Discontinue metformin hydrochloride tablets and metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m 2 ; in patients with a history of liver disease, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart metformin hydrochloride tablets and metformin hydrochloride extended-release tablets if renal function is stable.
