Drug Catalog - Product Detail
Methocarbamol Tab 500 MG 500 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 76385-0123-50 | BAYSHORE PHARMACEUTICALS | 500 | 500MG | TABLET |
PACKAGE FILES


Generic Name
METHOCARBAMOL
Substance Name
METHOCARBAMOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA208507
Description
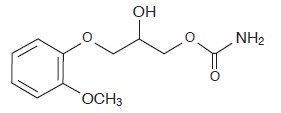
DESCRIPTION Methocarbamol Tablets USP, a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties. The chemical name of methocarbamol is 3 - (2-methoxyphenoxy) -1, 2- propanediol 1-carbamate and has the empirical formula C 11 H 15 NO 5 . Its molecular weight is 241.24. The structural formula is shown below. Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane. Methocarbamol Tablets USP, 500 mg is available as a light orange colored, round, film-coated tablets, engraved with 'B134' on one side and scored on the other side, containing 500 mg of methocarbamol, USP for oral administration. The inactive ingredients present are corn starch, low substituted hydroxypropyl cellulose, hydroxyprolyl cellulose, sodium starch glycolate, povidone, sodium lauryl sulfate, colloidal silicon dioxide, stearic acid, magnesium stearate, and purified water. Methocarbamol Tablets USP, 500 mg contains Opadry 13H530000 (Orange) (hypromellose, titanium dioxide, propylene glycol, FD&C yellow #6/Sunset Yellow FCF Aluminum Lake, polysorbate 20) as coating material. Methocarbamol Tablets USP, 750 mg is available as an orange colored, capsule shaped, film coated tablets, engraved with 'B135' on one side and plain on the other side, containing 750 mg of methocarbamol, USP for oral administration. It contains Opadry 13H530001 (Orange) as coating material. The inactive ingredients present are corn starch, low substituted hydroxypropyl cellulose, hydroxyprolyl cellulose, sodium starch glycolate, povidone, sodium lauryl sulfate, colloidal silicon dioxide, stearic acid, magnesium stearate, and purified water. Methocarbamol Tablets USP, 750 mg contain Opadry 13H530001 (Orange) (hypromellose, titanium dioxide, propylene glycol, D&C Yellow #10 Aluminum Lake, FD&C yellow #6/Sunset Yellow FCC Aluminum Lake, polysorbate 20) as coating material. structure
How Supplied
HOW SUPPLIED Methocarbamol Tablets USP, 500 mg are light orange colored, round, film-coated tablets, engraved with 'B134' on one side and scored on the other side. They are supplied as follows: Bottles of 100 NDC 76385-123-01 Bottles of 500 NDC 76385-123-50 Methocarbamol Tablets USP, 750 mg are orange colored, capsule shaped, film coated tablets, engraved with 'B135' on one side and plain on the other side. They are supplied as follows: Bottles of 100 NDC 76385-124-01 Bottles of 500 NDC 76385-124-50 Store at controlled room temperature, between 20°C and 25°C (68°F and 77°F). Dispense in tight container. Manufactured for Beximco Pharmaceuticals USA Inc. 4110 Regal Oaks Drive, P.O. Box 1060 Suwanee, GA 30024, USA Manufactured by BEXIMCO PHARMACEUTICALS LTD. 126, Kathaldia, Tongi, Gazipur, 1711, Bangladesh Distributed by: Bayshore Pharmaceuticals LLC Short Hills, NJ 07078 Last revised on 12/2017 5001397 111217
Indications & Usage
INDICATIONS AND USAGE Methocarbamol Tablets USP, 500 mg and 750 mg are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
Dosage and Administration
DOSAGE AND ADMINISTRATION Methocarbamol Tablets USP, 500 mg – Adults: Initial dosage: 3 tablets q.i.d. Maintenance dosage: 2 tablets q.i.d. Methocarbamol Tablets USP, 750 mg – Adults: Initial dosage: 2 tablets q.i.d. Maintenance dosage: 1 tablet q.4h. or 2 tablets t.i.d. Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered). Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
