Drug Catalog - Product Detail
Methylphenidate HCl Tab 20 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1802-01 | AMNEAL PHARMACEUTICALS | 100 | 20MG | TABLET |
PACKAGE FILES


Generic Name
METHYLPHENIDATE HYDROCHLORIDE
Substance Name
METHYLPHENIDATE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091159
Description
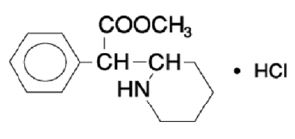
11 DESCRIPTION Methylphenidate hydrochloride tablets, USP contains methylphenidate hydrochloride USP, a CNS stimulant. It is available as tablets of 5 mg, 10 mg and 20 mg strengths for oral administration. Methylphenidate hydrochloride, USP is methyl α-phenyl-2-piperidineacetate hydrochloride, and its structural formula is: Methylphenidate hydrochloride, USP is a white to off-white powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol. Methylphenidate hydrochloride tablets, USP contain the following inactive ingredients: Lactose monohydrate, microcrystalline cellulose, stearic acid and FD&C Yellow No. 6 Aluminum Lake. 1
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Methylphenidate hydrochloride tablets USP, 5 mg are supplied as light orange to orange, round compressed tablets, debossed cor on one side and 237 on the other side. They are available as follows: Bottles of 100: NDC 0115-1800-01 Methylphenidate hydrochloride tablets USP, 10 mg are supplied as light orange to orange, round compressed tablets, debossed cor above bisect and 238 below the bisect on one side and plain on the other side. They are available as follows: Bottles of 100: NDC 0115-1801-01 Methylphenidate hydrochloride tablets USP, 20 mg are supplied as light orange to orange, round compressed tablets, debossed cor over 239 on one side and bisect on the other side. They are available as follows: Bottles of 100: NDC 0115-1802-01 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light. Dispense in tight, light-resistant container (USP).
Indications & Usage
1 INDICATIONS AND USAGE Methylphenidate hydrochloride tablets are indicated for the treatment of: Attention Deficit Hyperactivity Disorders (ADHD) in pediatric patients 6 years and older and adults Narcolepsy Methylphenidate hydrochloride tablets are a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorders (ADHD) and Narcolepsy ( 1 ).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Pediatric Patients 6 Years and Older: Start with 5 mg twice daily (before breakfast and lunch), titrating the dose weekly in 5 mg to 10 mg increments. Dosages above 60 mg/day are not recommended (2.2) . Adults: Average daily dosage is 20 mg to 30 mg, administered 2 or 3 times daily, preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg (2.2) . 2.1 Pre-treatment Screening Prior to treating patients with methylphenidate hydrochloride tablets, assess: for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2) ] . the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating methylphenidate hydrochloride tablets [see Warnings and Precautions (5.10) ] . 2.2 General Dosing Information Pediatric Patients 6 years and Older: Start with 5 mg orally twice daily (before breakfast and lunch). Increase dosage gradually, in increments of 5 mg to 10 mg weekly. Daily dosage above 60 mg is not recommended. Adults: Average dosage is 20 mg to 30 mg daily. Administer orally in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Maximum total daily dosage is 60 mg. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m. 2.3 Dosage Reduction and Discontinuation If paradoxical worsening of symptoms or other adverse reactions occur, reduce the dosage, or, if necessary, discontinue methylphenidate hydrochloride tablets. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
