Drug Catalog - Product Detail
METOPROLOL SUCCINATE ER 25MG TABS 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45963-0709-11 | ACTAVIS | 100 | 25MG | TABLET |
PACKAGE FILES












Generic Name
METOPROLOL SUCCINATE
Substance Name
METOPROLOL SUCCINATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204161
Description
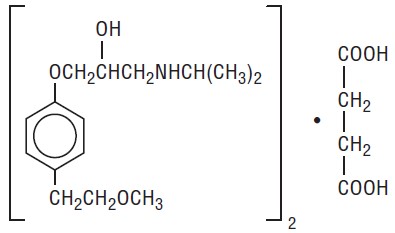
11 DESCRIPTION Metoprolol succinate, USP is a beta 1 -selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate extended-release tablets, USP have been formulated to provide a controlled and predictable release of metoprolol for once-daily administration. The tablets comprise a multiple unit system containing metoprolol succinate, USP in a multitude of controlled release pellets. Each pellet acts as a separate drug delivery unit and is designed to deliver metoprolol continuously over the dosage interval. The tablets contain 23.75, 47.5, 95 and 190 mg of metoprolol succinate, USP equivalent to 25, 50, 100 and 200 mg of metoprolol tartrate, USP, respectively. Its chemical name is (±)1-(isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol succinate (2:1) (salt). Its structural formula is: Metoprolol succinate, USP is a white crystalline powder with a molecular weight of 652.8. It is freely soluble in water; soluble in methanol; sparingly soluble in ethanol; slightly soluble in dichloromethane and 2-propanol; practically insoluble in ethyl-acetate, acetone, diethylether and heptane. Inactive ingredients: acetyltributyl citrate, caprylic/capric triglyceride, colloidal silicon dioxide, copovidone, crospovidone, denatured alcohol, ethylcellulose, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 3350, polyethylene glycol 6000, talc and titanium dioxide. USP dissolution test pending. 1
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Metoprolol succinate extended-release tablets, USP are supplied as follows: 25 mg – Each white to off-white, capsule shaped, film-coated tablet, debossed with on one side and A9 on the other side and scored on both sides contains 23.75 mg of metoprolol succinate, USP equivalent to 25 mg of metoprolol tartrate, USP. Tablets are supplied in bottles of 100 (NDC 45963-709-11) and bottles of 1,000 (NDC 45963-709-96). 50 mg – Each white to off-white, capsule shaped, film-coated tablet, debossed with and 676 on one side and scored on the other side contains 47.5 mg of metoprolol succinate, USP equivalent to 50 mg of metoprolol tartrate, USP. Tablets are supplied in bottles of 100 (NDC 45963-676-11) and bottles of 1,000 (NDC 45963-676-96). 100 mg – Each white to off-white, capsule shaped, film-coated tablet, debossed with and 677 on one side and scored on the other side contains 95 mg of metoprolol succinate, USP equivalent to 100 mg of metoprolol tartrate, USP. Tablets are supplied in bottles of 100 (NDC 45963-677-11) and bottles of 1,000 (NDC 45963-677-96). 200 mg – Each white to off-white, capsule shaped, film-coated tablet, debossed with and 678 on one side and scored on the other side contains 190 mg of metoprolol succinate, USP equivalent to 200 mg of metoprolol tartrate, USP. Tablets are supplied in bottles of 100 (NDC 45963-678-11). Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. 1 1 1 1
Indications & Usage
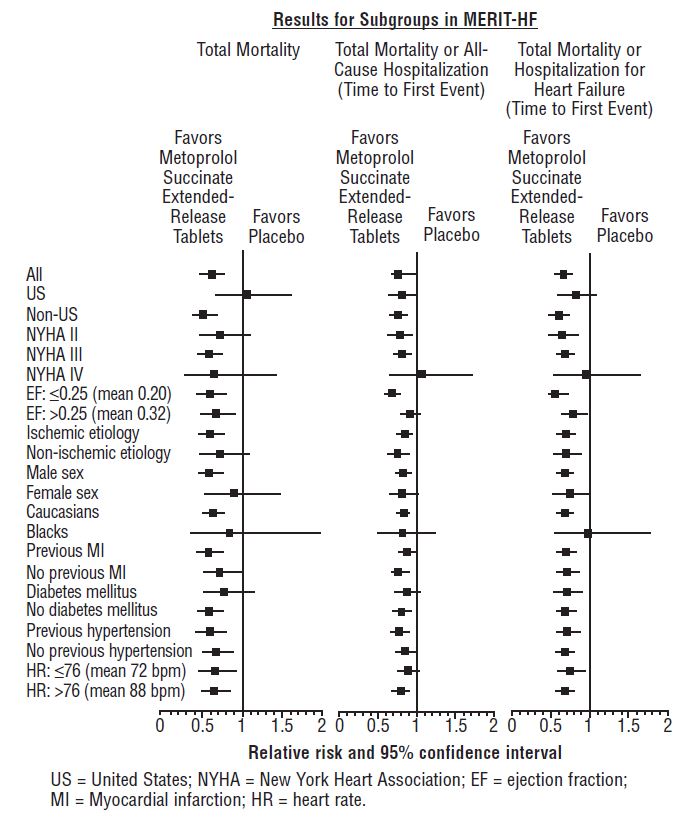
1 INDICATIONS AND USAGE Metoprolol succinate extended-release tablets are a beta-adrenergic blocker indicated for the treatment of: Hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1.1 ) Angina Pectoris. ( 1.2 ) Heart Failure, to reduce the risk of cardiovascular mortality and heart failure hospitalizations in patients with heart failure. ( 1.3 ) 1.1 Hypertension Metoprolol succinate extended-release tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including metoprolol. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Metoprolol succinate extended-release tablets may be administered with other antihypertensive agents. 1.2 Angina Pectoris Metoprolol succinate extended-release tablets are indicated in the long-term treatment of angina pectoris, to reduce angina attacks and to improve exercise tolerance. 1.3 Heart Failure Metoprolol succinate extended-release tablets are indicated to reduce the risk of cardiovascular mortality and heart-failure hospitalization in patients with heart failure.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administer once daily. Titrate at weekly or longer intervals as needed and tolerated. ( 2 ) Hypertension: Starting dose is 25 to 100 mg. ( 2.1 ) Angina Pectoris: Starting dose is 100 mg. ( 2.2 ) Heart Failure: Starting dose is 12.5 or 25 mg. ( 2.3 ) Switching from immediate-release metoprolol to metoprolol succinate extended-release tablets: use the same total daily dose of metoprolol succinate extended-release tablets. ( 2 ) 2.1 Hypertension Adults: The usual initial dosage is 25 to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after 1 week of therapy. Dosages above 400 mg per day have not been studied. Pediatric Hypertensive Patients ≥ 6 Years of age: The recommended starting dose of metoprolol succinate extended-release tablets is 1 mg/kg once daily, but the maximum initial dose should not exceed 50 mg once daily. Adjust dosage according to blood pressure response. Doses above 2 mg/kg (or in excess of 200 mg) once daily have not been studied in pediatric patients [see Use in Specific Populations ( 8.4 ) and Clinical Pharmacology ( 12.3 )] . Metoprolol succinate extended-release tablets have not been studied in pediatric patients < 6 years of age [see Use in Specific Populations ( 8.4 )] . 2.2 Angina Pectoris Individualize the dosage of metoprolol succinate extended-release tablets. The usual initial dosage is 100 mg daily, given in a single dose. Gradually increase the dosage at weekly intervals until optimum clinical response has been obtained or there is a pronounced slowing of the heart rate. Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, reduce the dosage gradually over a period of 1 to 2 weeks [see Warnings and Precautions ( 5 )] . 2.3 Heart Failure Dosage must be individualized and closely monitored during up-titration. Prior to initiation of metoprolol succinate extended-release tablets, stabilize the dose of other heart failure drug therapy. The recommended starting dose of metoprolol succinate extended-release tablets is 25 mg once daily for two weeks in patients with NYHA Class II heart failure and 12.5 mg once daily in patients with more severe heart failure. Double the dose every two weeks to the highest dosage level tolerated by the patient or up to 200 mg of metoprolol succinate extended-release tablets. Initial difficulty with titration should not preclude later attempts to introduce metoprolol succinate extended-release tablets. If patients experience symptomatic bradycardia, reduce the dose of metoprolol succinate extended-release tablets. If transient worsening of heart failure occurs, consider treating with increased doses of diuretics, lowering the dose of metoprolol succinate extended-release tablets or temporarily discontinuing it. The dose of metoprolol succinate extended-release tablets should not be increased until symptoms of worsening heart failure have been stabilized. 2.4 Administration Metoprolol succinate extended-release tablets are scored and can be divided; however, do not crush or chew the whole or half tablet.
