Drug Catalog - Product Detail
MISOPROSTOL TAB 200MCG 60CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43386-0161-06 | LUPIN PHARMACEUTICALS | 60 | 200MCG | TABLET |
PACKAGE FILES
Generic Name
MISOPROSTOL
Substance Name
MISOPROSTOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091667
Description
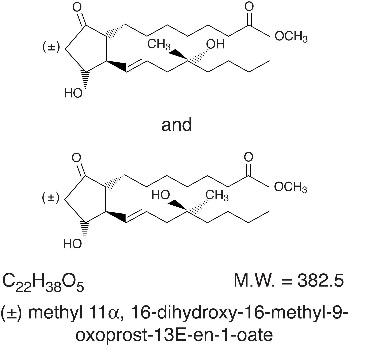
DESCRIPTION Misoprostol oral tablets contain either 100 mcg or 200 mcg of misoprostol, a synthetic prostaglandin E1 analog. Misoprostol contains approximately equal amounts of the two diastereomers presented below with their enantiomers indicated by (±): Misoprostol is a water-soluble, viscous liquid. Inactive ingredients of tablets are hydrogenated castor oil, microcrystalline cellulose, and crospovidone 9023f150-figure-01
How Supplied
HOW SUPPLIED Misoprostol Tablets 100-mcg tablets are round, white flat-faced beveled edge tablets, debossed "160" on one side and "n" on other side; supplied as: Bottles of 60: 43386-160-06 Bottles of 120: 43386-160-12 Misoprostol Tablets 200-mcg tablets are round, white flat-faced beveled edge bisected tablets, debossed "161" above the bisect and "n" below the bisect and plain on the other side; supplied as: Bottles of 60: 43386-161-06 Bottles of 100: 43386-161-01 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Store in a dry area.
Indications & Usage
INDICATIONS AND USAGE Misoprostol is indicated for reducing the risk of NSAID (nonsteroidal anti-inflammatory drugs, including aspirin)–induced gastric ulcers in patients at high risk of complications from gastric ulcer, e.g., the elderly and patients with concomitant debilitating disease, as well as patients at high risk of developing gastric ulceration, such as patients with a history of ulcer. Misoprostol Tablet has not been shown to reduce the risk of duodenal ulcers in patients taking NSAIDs. Misoprostol Tablets should be taken for the duration of NSAID therapy. Misoprostol Tablets has been shown to reduce the risk of gastric ulcers in controlled studies of 3 months' duration. It had no effect, compared to placebo, on gastrointestinal pain or discomfort associated with NSAID use.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended adult oral dose of Misoprostol Tablets for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used. (See Clinical Pharmacology : Clinical studies .) Misoprostol Tablets should be taken for the duration of NSAID therapy as prescribed by the physician. Misoprostol Tablets should be taken with a meal, and the last dose of the day should be at bedtime.
