Drug Catalog - Product Detail
MOEXIPRIL HCL TB 15MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0208-01 | GLENMARK PHARMACEUTICALS | 100 | 15MG | TABLET |
PACKAGE FILES



Generic Name
MOEXIPRIL HYDROCHLORIDE
Substance Name
MOEXIPRIL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090416
Description
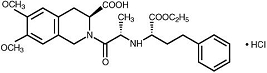
DESCRIPTION Moexipril hydrochloride USP, the hydrochloride salt of moexipril, has the empirical formula C 27 H 34 N 2 O 7 •HCl and a molecular weight of 535.04. It is chemically described as [3S-[2[R*(R*)],3R*]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid, monohydrochloride. It is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat and its structural formula is: Moexipril hydrochloride USP is a fine white to off-white powder. It is soluble (about 10% weight-to-volume) in distilled water at room temperature. Moexipril hydrochloride tablets USP are supplied as scored, coated tablets containing 7.5 mg and 15 mg of moexipril hydrochloride USP for oral administration. In addition to the active ingredient, moexipril hydrochloride USP, the tablet core contains the following inactive ingredients: crospovidone, lactose monohydrate, magnesium oxide, magnesium stearate and povidone. The film coating contains: hypromellose, hydroxypropyl cellulose, titanium dioxide, polyethylene glycol 6000, magnesium stearate, ferric oxide red, ferric oxide black and ferric oxide yellow (15 mg tablet only). structural-formula
How Supplied
HOW SUPPLIED Moexipril hydrochloride tablets USP 7.5 mg are peach, round, biconvex, film coated tablets with ‘G' and breakline engraved on one side and ‘209’ on the other side. Bottles of 90 NDC 68462-209-90 Bottles of 100 NDC 68462-209-01 Bottles of 1000 NDC 68462-209-10 Moexipril hydrochloride tablets USP 15 mg are brown, round, biconvex, film coated tablets with ‘G' and breakline engraved on one side and ‘208’ on the other side. Bottles of 90 NDC 68462-208-90 Bottles of 100 NDC 68462-208-01 Bottles of 1000 NDC 68462-208-10
Indications & Usage
INDICATIONS AND USAGE Moexipril hydrochloride tablets are indicated for treatment of patients with hypertension. They may be used alone or in combination with thiazide diuretics. In using moexipril hydrochloride tablets, consideration should be given to the fact that another ACE inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen-vascular disease. Available data are insufficient to show that moexipril hydrochloride tablets do not have a similar risk (see WARNINGS ). In considering use of moexipril hydrochloride tablets, it should be noted that in controlled trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, ACE inhibitors (for which adequate data are available) cause a higher rate of angioedema in black than in non-black patients (see WARNINGS, Angioedema ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension The recommended initial dose of moexipril hydrochloride tablets in patients not receiving diuretics is 7.5 mg, one hour prior to meals, once daily. Dosage should be adjusted according to blood pressure response. The antihypertensive effect of moexipril hydrochloride tablets may diminish towards the end of the dosing interval. Blood pressure should, therefore, be measured just prior to dosing to determine whether satisfactory blood pressure control is obtained. If control is not adequate, increased dose or divided dosing can be tried. The recommended dose range is 7.5 to 30 mg daily, administered in one or two divided doses one hour before meals. Total daily doses above 60 mg a day have not been studied in hypertensive patients. In patients who are currently being treated with a diuretic, symptomatic hypotension may occasionally occur following the initial dose of moexipril hydrochloride tablets. The diuretic should, if possible, be discontinued for 2 to 3 days before therapy with moexipril hydrochloride tablets is begun, to reduce the likelihood of hypotension (see WARNINGS ). If the patient’s blood pressure is not controlled with moexipril hydrochloride tablets alone, diuretic therapy may then be reinstituted. If diuretic therapy cannot be discontinued, an initial dose of 3.75 mg of moexipril hydrochloride tablets should be used with medical supervision until blood pressure has stabilized (see WARNINGS and PRECAUTIONS, Drug Interactions ). Dosage Adjustment in Renal Impairment For patients with a creatinine clearance ≤40 mL/min/1.73 m 2 , an initial dose of 3.75 mg once daily should be given cautiously. Doses may be titrated upward to a maximum daily dose of 15 mg.
