Drug Catalog - Product Detail
MOMETASONE FUROATE TOPICAL SOLUTION, USP SOL 0.001 30ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0118-59 | PADAGIS | 30 | 0.1% | SOLUTION |
PACKAGE FILES

Generic Name
MOMETASONE FUROATE
Substance Name
MOMETASONE FUROATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA077180
Description
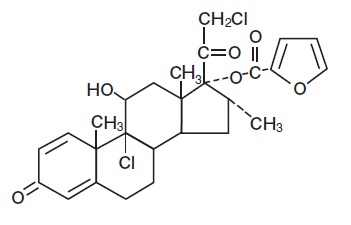
11 DESCRIPTION Mometasone Furoate Topical Solution USP, 0.1% contains mometasone furoate for topical use. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity. Chemically, mometasone furoate is 9α, 21-dichloro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C 27 H 30 Cl 2 O 6 , a molecular weight of 521.4 and the following structural formula: Mometasone furoate is a white to off-white powder insoluble in water, freely soluble in acetone and in methylene chloride and sparingly soluble in heptane. Each gram of Mometasone Furoate Topical Solution USP, 0.1% contains 1 mg mometasone furoate in a colorless, clear to translucent solution base of isopropyl alcohol (40%), hexylene glycol, hydroxypropyl cellulose, sodium phosphate monobasic anhydrous, purified water, glycerin and oleic acid. May also contain phosphoric acid used to adjust the pH to approximately 4.5. 5d8-chemical-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Mometasone Furoate Topical Solution USP, 0.1% is colorless, clear to translucent and supplied in 30-mL (27.5 gram) (NDC 45802- 118 -59) and 60-mL (55 gram) (NDC 45802- 118 -46) bottles; boxes of one. Store Mometasone Furoate Topical Solution, 0.1% at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Mometasone Furoate Topical Solution 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 12 years of age or older. Mometasone Furoate Topical Solution 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients ≥ 12 years of age.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Apply a few drops of Mometasone Furoate Topical Solution 0.1% to the affected skin areas once daily and massage lightly until it disappears. Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]. Do not use Mometasone Furoate Topical Solution, 0.1% with occlusive dressings unless directed by a physician. Do not apply Mometasone Furoate Topical Solution, 0.1% in the diaper area if the patient requires diapers or plastic pants, as these garments constitute occlusive dressing. Mometasone Furoate Topical Solution 0.1% is for topical use only. It is not for oral, ophthalmic, or intravaginal use. Avoid use on the face, groin, or axillae. Avoid contact with eyes. Wash hands after each application. • Apply a few drops to the affected skin areas once daily and massage lightly until it disappears. (2) • Discontinue therapy when control is achieved. (2) • If no improvement is seen within 2 weeks, reassess diagnosis. (2) • Do not use with occlusive dressings unless directed by a physician. (2)
