Drug Catalog - Product Detail
NABUMETONE 500MG TABLETS 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-3670-05 | ACTAVIS PHARMA | 500 | 500MG | TABLET |
PACKAGE FILES


Generic Name
NABUMETONE
Substance Name
NABUMETONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091083
Description
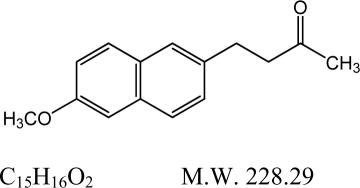
DESCRIPTION Nabumetone, USP is a naphthylalkanone designated chemically as 4-(6-methoxy-2-naphthalenyl)-2-butanone. It has the following structure: Nabumetone, USP is a white to off-white crystalline substance. It is nonacidic and practically insoluble in water, but soluble in alcohol and most organic solvents. It has an n-octanol: phosphate buffer partition coefficient of 2,400 at pH 7.4. Each tablet, for oral administration, contains either 500 mg or 750 mg of nabumetone, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, povidone, sodium lauryl sulfate, sodium starch glycolate, titanium dioxide and triacetin. The 500 mg tablets also contain talc and the 750 mg tablets also contain iron oxide red. Structure
How Supplied
HOW SUPPLIED Nabumetone tablets USP, 500 mg are white, oval-shaped, film-coated, biconvex tablets debossed with “ 3670 ” on one side of the tablet and plain on the other side. They are available in bottles of 100 and 500. NDC 0591-3670-01...............................bottles of 100 NDC 0591-3670-05...............................bottles of 500 Nabumetone tablets USP, 750 mg are pink, oval-shaped, film-coated, biconvex tablets debossed with “ 3671 ” on one side of the tablet and plain on the other side. They are available in bottles of 100 and 500. NDC 0591-3671-01...............................bottles of 100 NDC 0591-3671-05...............................bottles of 500 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Dispense with Medication Guide available at: www.tevausa.com/medguides Manufactured In India By: Watson Pharma Private Ltd. Verna, Salcette Goa 403 722 INDIA Manufactured For: Teva Pharmaceuticals Parsippany, NJ 07054 Rev. D 2/2022
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of nabumetone tablets and other treatment options before deciding to use nabumetone tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). Nabumetone tablets are indicated for relief of signs and symptoms of osteoarthritis and rheumatoid arthritis.
Dosage and Administration
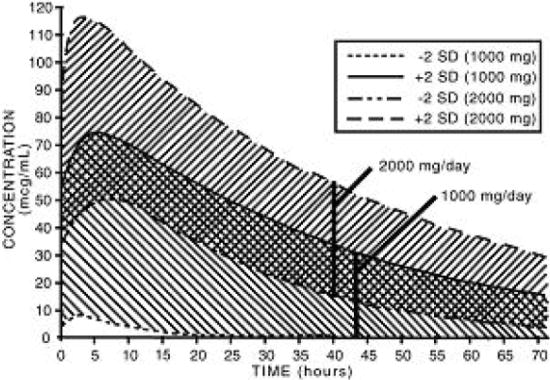
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of nabumetone tablets and other treatment options before deciding to use nabumetone tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with nabumetone tablets, the dose and frequency should be adjusted to suit an individual patient's needs. Osteoarthritis and Rheumatoid Arthritis The recommended starting dose is 1,000 mg taken as a single dose with or without food. Some patients may obtain more symptomatic relief from 1,500 mg to 2,000 mg per day. Nabumetone tablets can be given in either a single or twice-daily dose. Dosages greater than 2,000 mg per day have not been studied. The lowest effective dose should be used for chronic treatment (see WARNINGS , Renal Effects ). Patients weighing under 50 kg may be less likely to require dosages beyond 1,000 mg; therefore, after observing the response to initial therapy, the dose should be adjusted to meet individual patients' requirements.
