Drug Catalog - Product Detail
NABUMETONE 750MG TB 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 50228-0466-01 | SCIEGEN PHARMACEUTICALS | 100 | 750MG | TABLET |
PACKAGE FILES




Generic Name
NABUMETONE
Substance Name
NABUMETONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078420
Description
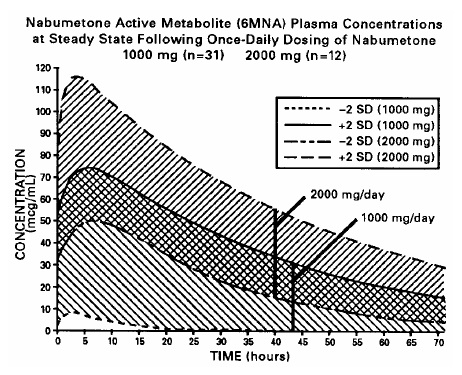
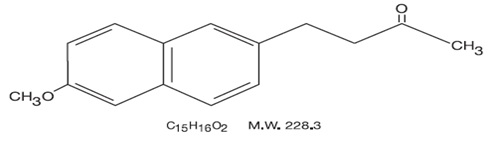
DESCRIPTION Nabumetone is a naphthylalkanone designated chemically as 4-(6-methoxy-2- naphthalenyl)-2- butanone. It has the following structure: Nabumetone, USP is a white or almost white crystalline substance with a molecular weight of 228.3. It is nonacidic, freely soluble in acetone, sparingly soluble in alcohol and in methanol, practically insoluble in water. It has an n-octanol:phosphate buffer partition coefficient of 2400 at pH 7.4. Each tablet, for oral administration contains either 500 mg or 750 mg of nabumetone, USP. In addition, each tablet contains the following inactive ingredients: hypromellose, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate, polyethylene glycol and titanium dioxide. structure1
How Supplied
HOW SUPPLIED Nabumetone Tablets, USP: Nabumetone tablets USP, 500 mg are white colored, oval shaped, biconvex, film coated tablets, debossed with ‘SG’ on one side ‘465’ on other side. Bottles of 30 NDC 50228-465-30 Bottles of 100 NDC 50228-465-01 Bottles of 500 NDC 50228-465-05 Nabumetone tablets USP, 750 mg are white colored, oval shaped, biconvex, film coated tablets, debossed with ‘SG’ on one side ‘466’ on other side. Bottles of 30 NDC 50228-466-30 Bottles of 100 NDC 50228-466-01 Bottles of 500 NDC 50228-466-05 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Rx Only Manufactured by: ScieGen Pharmaceuticals, Inc. Hauppauge, NY 11788 Rev: 8/2024
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of nabumetone tablets, USP and other treatment options before deciding to use nabumetone tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). Nabumetone tablets, USP are indicated for relief of signs and symptoms of osteoarthritis and rheumatoid arthritis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of nabumetone tablets and other treatment options before deciding to use nabumetone tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with nabumetone tablets, the dose and frequency should be adjusted to suit an individual patient’s needs. Osteoarthritis and Rheumatoid Arthritis: The recommended starting dose is 1,000 mg taken as a single dose with or without food. Some patients may obtain more symptomatic relief from 1,500 mg to 2,000 mg per day. Nabumetone can be given in either a single or twice-daily dose. Dosages greater than 2,000 mg per day have not been studied. The lowest effective dose should be used for chronic treatment (see WARNINGS , Renal Effects ). Patients weighing under 50 kg may be less likely to require dosages beyond 1,000 mg; therefore, after observing the response to initial therapy, the dose should be adjusted to meet individual patients’ requirements.
