Drug Catalog - Product Detail
NEOMYCIN/POLYMYXIN B/DEXAMETH OPHTH SUSP SUSP .35/10/.1MU/ML 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0830-60 | BAUSCH HEALTH US | 5 | 3.5-10000-0.1 | SUSPENSION |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
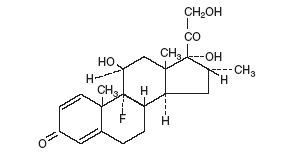
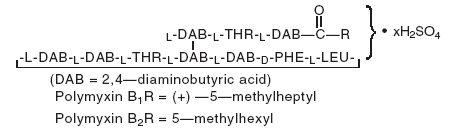
DESCRIPTION: Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Suspension USP is a multiple dose anti-infective steroid combination in a sterile suspension for topical application. The active ingredient, Dexamethasone, is represented by the following structural formula: C 22 H 29 FO 5 Mol. Wt. 392.47 Chemical Name: Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11β,16α)-. Neomycin Sulfate is the sulfate salt of neomycin B and C which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent to not less than 600 micrograms of neomycin base per milligram, calculated on an anhydrous basis. The structural formula is: Polymyxin B Sulfate is the sulfate salt of polymyxin B 1 and B 2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per milligram, calculated on an anhydrous basis. The structural formula is: Each mL Contains: ACTIVES: Neomycin Sulfate (equivalent to 3.5 mg Neomycin), Polymyxin B Sulfate equal to 10,000 polymyxin B units, Dexamethasone 1 mg (0.1%); INACTIVES: Sodium Chloride, Hypromellose, Polysorbate 20, Purified Water. Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (3.5 – 6.0). PRESERVATIVE ADDED: Benzalkonium Chloride 0.004% Dexamethasone (structural formula) Neomycin Sulfate (structural formula) Polymyxin B Sulfate (structural formula)
How Supplied
HOW SUPPLIED: Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Suspension USP is supplied in a controlled drop tip bottle in the following size: 5 mL - Prod. No. 04107 DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT. FOR OPHTHALMIC USE ONLY Storage Store between 15°-30° C (59°-86° F). Store upright. KEEP OUT OF REACH OF CHILDREN. Revised September 2008 Bausch & Lomb Incorporated Tampa, FL 33637 ©Bausch & Lomb Incorporated 9114201 (Folded) 9114301 (Flat) H.J. Harkins Company, Inc. 513 Sandydale Drive Nipomo, CA 93444
Indications & Usage
INDICATIONS AND USAGE: For steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where bacterial infection or a risk of bacterial ocular infection exists. Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitis is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies. The use of a combination drug with an anti-infective component is indicated where the risk of infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye. The particular anti-infective drug in this product is active against the following common bacterial eye pathogens: Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Klebsiella/Enterobacter species, Neisseria species, Pseudomonas aeruginosa . This product does not provide adequate coverage against Serratia marcescens , and Streptococci, including Streptococcus pneumoniae .
Dosage and Administration
DOSAGE AND ADMINISTRATION: One or two drops topically in the conjunctival sac(s). In severe disease, drops may be used hourly, being tapered to discontinuation as the inflammation subsides. In mild disease, drops may be used up to four to six times daily. Not more than 20 mL should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above. SHAKE WELL BEFORE USING.
