Drug Catalog - Product Detail
NISOLDIPINE ER TB 17MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 66993-0473-02 | PRASCO LABORATORIES | 100 | 17MG | TABLET |
PACKAGE FILES
Generic Name
NISOLDIPINE
Substance Name
NISOLDIPINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA020356
Description
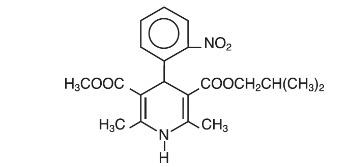
DESCRIPTION Nisoldipine is an extended release tablet dosage form of the dihydropyridine calcium channel blocker nisoldipine. Nisoldipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, methyl 2-methylpropyl ester, C 20 H 24 N 2 O 6 , and has the structural formula: Nisoldipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 388.4. Nisoldipine tablets comprise three layers: a top barrier layer, a middle layer containing nisoldipine, and a bottom barrier layer. The erodible barrier layers and the hydrogel middle layer provide for the controlled release of the drug. Nisoldipine tablets contain either 8.5, 17, or 34 mg of nisoldipine for once-a-day oral administration. Inactive ingredients in the formulation include: Hypromellose, hypromellose phthalate, lactose, glyceryl behenate, povidone, magnesium stearate, silicon dioxide, methacrylic acid copolymer, and sodium lauryl sulfate. Inactive ingredients in the film coating include: polydextrose, titanium dioxide, hypromellose, polyethylene glycol, iron oxide, and carnauba wax. Additionally, the 17 mg formulation contains FD&C Yellow #5. chem
How Supplied
HOW SUPPLIED Nisoldipine extended release tablets are supplied as 8.5 mg and 17 mg round film coated tablets and 34 mg elliptic film coated tablets. The different strengths can be identified as follows: Strength Color Markings 8.5 mg Oyster SCI 500 17 mg Yellow Cream SCI 501 34 mg Burnt Orange SCI 503 Nisoldipine Tablets are supplied in bottles of 100: NDC Code Strength 66993-472-02 8.5 mg 66993-473-02 17 mg 66993-475-02 34 mg Protect from light and moisture. Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant containers. Rx only Manufactured for: Prasco Laboratories Mason, OH 45040 USA Rev 10/2021 362168
Indications & Usage
INDICATIONS AND USAGE Nisoldipine is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Dosage and Administration
DOSAGE AND ADMINISTRATION The dosage of Nisoldipine must be adjusted to each patient’s needs. Therapy usually should be initiated with 17 mg orally once daily, then increased by 8.5 mg per week or longer intervals, to attain adequate control of blood pressure. Usual maintenance dosage is 17 to 34 mg once daily. Blood pressure response increases over the 8.5 - 34 mg daily dose range but adverse event rates also increase. Doses beyond 34 mg once daily are not recommended. Nisoldipine has been used safely with diuretics, ACE inhibitors, and beta-blocking agents. Patients over age 65, or patients with impaired liver function, are expected to develop higher plasma concentrations of nisoldipine. Their blood pressure should be monitored closely during any dosage adjustment. A starting dose not exceeding 8.5 mg daily is recommended in these patient groups. Nisoldipine tablets should be administered orally once daily. Nisoldipine should be taken on an empty stomach (1 hour before or 2 hours after a meal). Grapefruit products should be avoided before and after dosing. Nisoldipine is an extended release dosage form and tablets should be swallowed whole, not bitten, divided or crushed.
