Drug Catalog - Product Detail
Norethindrone Acetate 5mg 50 Tablet
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0475-05 | AMNEAL PHARMACEUTICALS | 50 | 5MG | TABLET |
PACKAGE FILES

Generic Name
NORETHINDRONE
Substance Name
NORETHINDRONE ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200275
Description
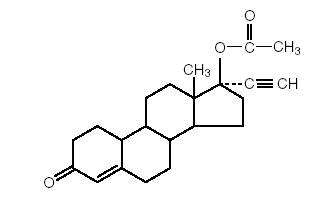
DESCRIPTION Norethindrone acetate tablets, USP - 5 mg oral tablets. Norethindrone acetate, USP (17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate), a synthetic, orally active progestin, is the acetic acid ester of norethindrone, USP. It is a white, or creamy white, crystalline powder. Norethindrone acetate tablets, USP contain the following inactive ingredients: lactose, magnesium stearate, and microcrystalline cellulose. 898a98a2-figure-01
How Supplied
HOW SUPPLIED Norethindrone acetate tablets, USP, 5 mg, are supplied as white to off-white oval, biconvex tablets debossed with “AN” bisect “475” on one side and plain on the other side. They are available as follows: Bottles of 50: NDC 65162-475-05 Bottles of 90: NDC 65162-475-09 Bottles of 500: NDC 65162-475-50 Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Distributed by: Amneal Pharmaceuticals Bridgewater, NJ 08807 Rev. 08-2023-01
Indications & Usage
INDICATIONS AND USAGE Norethindrone acetate tablets, USP is indicated for the treatment of secondary amenorrhea, endometriosis, and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer. Norethindrone acetate tablets, USP are not intended, recommended or approved to be used with concomitant estrogen therapy in postmenopausal women for endometrial protection.
Dosage and Administration
DOSAGE AND ADMINISTRATION Therapy with norethindrone acetate tablets, USP must be adapted to the specific indications and therapeutic response of the individual patient. Secondary amenorrhea, abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology: 2.5 to 10 mg norethindrone acetate, USP may be given daily for 5 to 10 days to produce secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen. Progestin withdrawal bleeding usually occurs within three to seven days after discontinuing norethindrone acetate, USP therapy. Patients with a past history of recurrent episodes of abnormal uterine bleeding may benefit from planned menstrual cycling with norethindrone acetate tablets, USP. Endometriosis: Initial daily dosage of 5 mg norethindrone acetate, USP for two weeks. Dosage should be increased by 2.5 mg per day every two weeks until 15 mg per day of norethindrone acetate, USP is reached. Therapy may be held at this level for six to nine months or until annoying breakthrough bleeding demands temporary termination.
