Drug Catalog - Product Detail
NORETHINDRONE TABLETS TB 0.35MG 3X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-7272-53 | MYLAN | 28 | 0.35MG | TABLET |
PACKAGE FILES

Generic Name
NORETHINDRONE
Substance Name
NORETHINDRONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200980
Description
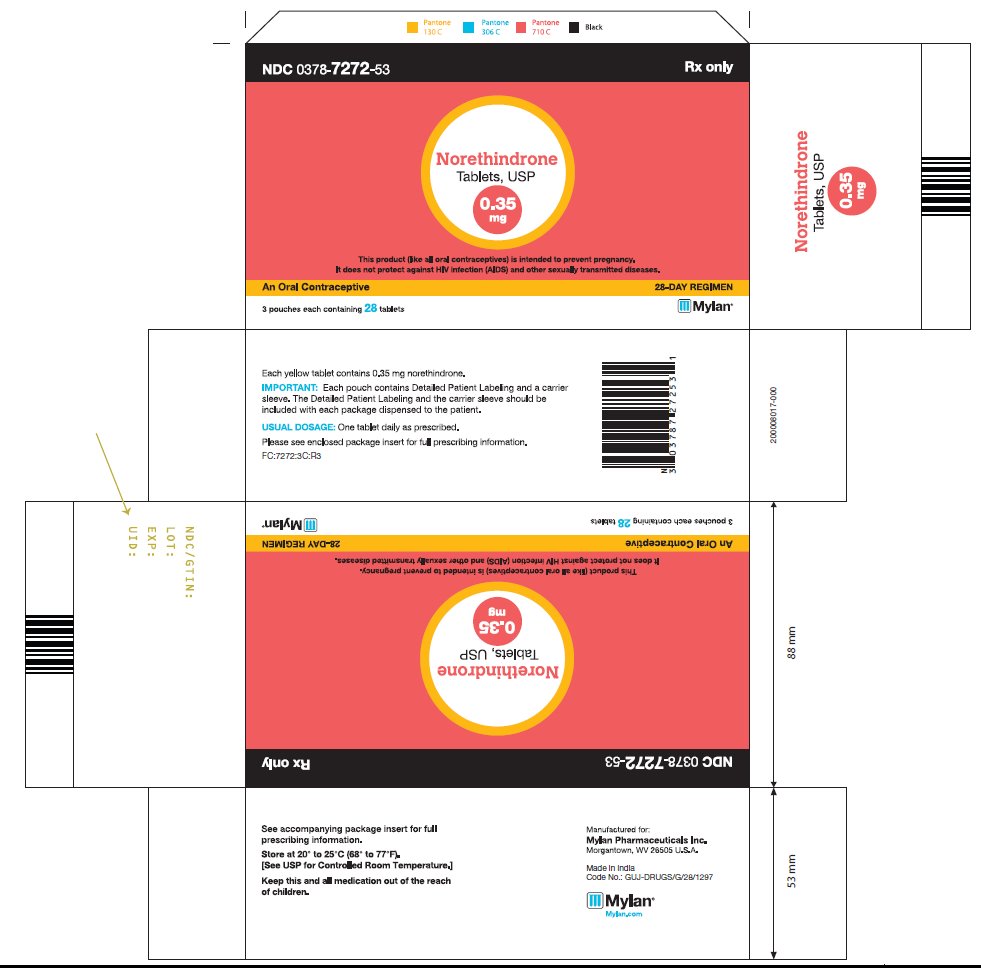
DESCRIPTION Norethindrone Tablets, USP Each tablet contains 0.35 mg norethindrone. Inactive ingredients include corn starch, D&C Yellow No. 10, ethyl cellulose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate and talc. Meets USP Dissolution Test 2 structure
How Supplied
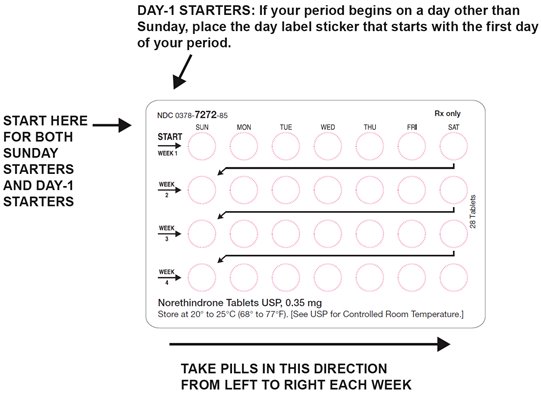
HOW SUPPLIED Norethindrone tablets USP, 0.35 mg are available in a blister pack containing 28 yellow, round, flat faced, beveled edge tablets, debossed 220 on one side and other side plain. Carton of 3 tri-laminated aluminium pouches (NDC 0378-7272-53), each pouch contains one blister pack of 28 tablets. Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Keep this and all medications out of the reach of children. REFERENCE 1. McCann M, and Potter L. Progestin-Only Oral Contraceptives: A Comprehensive Review. Contraception, 50:60 (Suppl. 1), December 1994. 2. Van Giersbergen PLM, Halabi A, Dingemanse J. Pharmacokinetic interaction between bosentan and the oral contraceptives norethisterone and ethinyl estradiol. Int J Clin Pharmacol Ther 2006;44(3):113-118. 3. Truitt ST, Fraser A, Gallo ME, Lopez LM, Grimes DA and Schulz KF. Combined hormonal versus nonhormonal versus progestin-only contraception in lactation (Review). The Cochrane Collaboration. 2007, Issue 3. 4. Halderman, LD and Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol.; 186 (6): 1250-1258. 5. Ostrea EM, Mantaring III JB, Silvestre MA. Drugs that affect the fetus and newborn infant via the placenta or breast milk. Pediatr Clin N Am; 51(2004): 539-579. 6. Cooke ID, Back DJ, Shroff NE: Norethisterone concentration in breast milk and infant and maternal plasma during ethynodiol diactetate administration. Contraception 1985; 31:611-21. 7. 2008 USPC Official:12/1/08-4/30/09, USP Monographs: Norethindrone Tablets (page 1 of 5).
Indications & Usage
INDICATIONS AND USAGE 1. Indications Progestin-only oral contraceptives are indicated for the prevention of pregnancy. 2. Efficacy If used perfectly, the first-year failure rate for progestin-only oral contraceptives is 0.3%. However, the typical failure rate is estimated to be closer to 9%, due to late or omitted pills. Table 1 lists the pregnancy rates for users of all major methods of contraception. Table 1: Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year. United States. Notes: Method (1) % of Women Experiencing an Unintended Pregnancy within the First Year of Use % of Women Continuing Use at One Year Among couples attempting to avoid pregnancy, the percentage who continue to use a method for 1 year. Typical Use Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. Estimates of the probability of pregnancy during the first year of typical use for spermicides, withdrawal, fertility awareness-based methods, the diaphragm, the male condom, the oral contraceptive pill, and Depo-Provera are taken from the 1995 National Survey of Family Growth corrected for underreporting of abortion; see the text for the derivation of estimates for the other methods. (2) Perfect Use Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. See the text for the derivation of the estimate for each method. (3) (4) No method The percentages becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within 1 year. This estimate was lowered slightly (to 85%) to represent the percentage who would become pregnant within 1 year among women now relying on reversible methods of contraception if they abandoned contraception altogether. 85 85 Spermicides Foams, creams, gels, vaginal suppositories, and vaginal film. 28 18 42 Fertility awareness-based methods 24 47 Standard Days method The Ovulation and TwoDay methods are based on evaluation of cervical mucus. The Standard Days method avoids intercourse on cycle days 8 through 19. The Symptothermal method is a double-check method based on evaluation of cervical mucus to determine the first fertile day and evaluation of cervical mucus and temperature to determine the last fertile day. 5 Two Day method 4 Ovulation method 3 Symptothermal 0.4 Withdrawal 22 4 46 Sponge 36 Parous women 24 20 Nulliparous women 12 9 Condom Without spermicides Female (fc) 21 5 41 Male 18 2 43 Diaphragm With spermicidal cream or jelly 12 6 57 Combined pill and progestin-only pill 9 0.3 67 Norelgestromin and ethinyl estradiol patch 9 0.3 67 NuvaRing 9 0.3 67 Depo-Provera 6 0.2 56 Intrauterine contraceptives ParaGard (copper T) 0.8 0.6 78 Mirena (LNg) 0.2 0.2 80 Implanon 0.05 0.05 84 Female sterilization 0.5 0.5 100 Male sterilization 0.15 0.10 100 Emergency Contraception: Emergency contraceptive pills or insertion of a copper intrauterine contraceptive after unprotected intercourse substantially reduces the risk of pregnancy. ella, Plan B One-Step and Next Choice are the only dedicated products specifically marketed for emergency contraception. The label for Plan B One-Step (one dose is 1 white pill) says to take the pill within 72 hours after unprotected intercourse. Research has shown that all of the brands listed here are effective when used within 120 hours after unprotected sex. The label for Next Choice (one dose is 1 peach pill) says to take 1 pill within 72 hours after unprotected intercourse and another pill 12 hours later. Research has shown that both pills can be taken at the same time with no decrease in efficacy or increase in side effects and that they are effective when used within 120 hours after unprotected sex. The FDA has in addition declared the following 19 brands of oral contraceptives to be safe and effective for emergency contraception: Ogestrel (1 dose is 2 white pills), Nordette (1 dose is 4 light-orange pills), Cryselle, Levora, Low-Ogestrel, Lo/Ovral, or Quasence (1 dose is 4 white pills), Jolessa, Portia, Seasonale or Trivora (1 dose is 4 pink pills), Seasonique (1 dose is 4 light-blue-green pills), Enpresse (one dose is 4 orange pills), Lessina (1 dose is 5 pink pills), Aviane or LoSeasonique (one dose is 5 orange pills), Lutera or Sronyx (one dose is 5 white pills), and Lybrel (one dose is 6 yellow pills). (See Chapter 6.). Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception. However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches 6 months of age. (See Chapter 18.) Source: Trussell J. Contraceptive Efficacy. In Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar M. Contraceptive Techology: Twentieth Revised Edition. New York NY: Ardent Media, 2011. Norethindrone tablets have not been studied for and are not indicated for use in emergency contraception.
Dosage and Administration
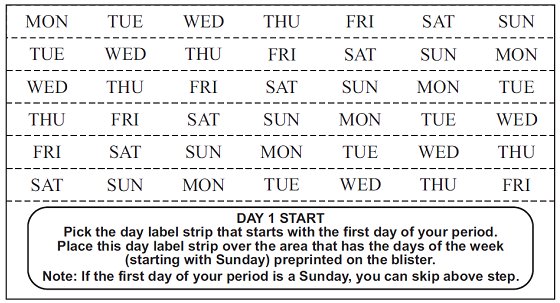
DOSAGE AND ADMINISTRATION To achieve maximum contraceptive effectiveness, norethindrone tablets must be taken exactly as directed. One tablet is taken every day, at the same time. Administration is continuous, with no interruption between pill packs. See Detailed Patient Labeling for detailed instruction.
