Drug Catalog - Product Detail
NORETHINDRONE/ETHINYL ESTRADIOL (TRI-LEGEST FE 28) TB 1 mg/20 mcg; 1 mg/30 mcg; 1 mg/35 mcg 5X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00555-9032-70 | TEVA PHARMACEUTICALS USA | 28 | 1-20/1-30/1-35MG-MCG | TABLET |
PACKAGE FILES





Generic Name
NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL
Substance Name
Product Type
HUMAN PRESCRIPTION DRUG
Route
Application Number
ANDA076105
Description
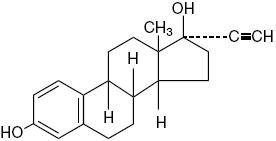
DESCRIPTION Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) is a graduated estrophasic combined oral contraceptive providing estrogen in a graduated sequence over a 21-day period with a constant dose of progestogen. Tri-Legest ® Fe provides for a continuous dosage regimen consisting of 21 oral contraceptive tablets and seven ferrous fumarate tablets. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. Each light pink tablet contains 1 mg norethindrone acetate [(17α)-17-(acetyloxy)-19-norpregna-4-en-20-yn-3-one] and 20 mcg ethinyl estradiol [(17α)-19-norpregna-1,3,5(10)-trien-20-yne-3,17-diol] Each light pink tablet contains the following inactive ingredients: calcium stearate, FD&C red no. 40 aluminum lake, lactose monohydrate, microcrystalline cellulose, pregelatinized corn starch, and sodium starch glycolate. Each light yellow tablet contains 1 mg norethindrone acetate and 30 mcg ethinyl estradiol. Each light yellow tablet contains the following inactive ingredients: calcium stearate, D&C yellow no.10 aluminum lake, lactose monohydrate, microcrystalline cellulose, pregelatinized corn starch, and sodium starch glycolate. Each light blue tablet contains 1 mg norethindrone acetate and 35 mcg ethinyl estradiol. Each light blue tablet contains the following inactive ingredients: calcium stearate, FD&C blue no. 2 aluminum lake, lactose monohydrate, microcrystalline cellulose, pregelatinized corn starch, and sodium starch glycolate. Each brown tablet contains crospovidone, ferrous fumarate, hydrogenated vegetable oil, and microcrystalline cellulose. The structural formulas are as follows: Norethindrone Acetate Ethinyl Estradiol C 22 H 28 O 3 Molecular Weight: 340.46 C 20 H 24 O 2 Molecular Weight: 296.40 Each Tri-Legest ® Fe tablet dispenser contains five light pink tablets, seven light yellow tablets, nine light blue tablets, and seven brown tablets. These tablets are to be taken in the following order: one light pink tablet each day for five days, then one light yellow tablet each day for seven days, followed by one light blue tablet each day for nine days, and then one brown tablet each day for seven days. 1 2
How Supplied
HOW SUPPLIED Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) is packaged in cartons of five tablet dispensers of 28 tablets each. Each tablet dispenser contains five light pink, round, flat-faced, beveled-edge, unscored tablets debossed with stylized b on one side and 711 on the other side each containing 1 mg of norethindrone acetate and 20 mcg of ethinyl estradiol; seven light yellow, round, flat-faced, beveled-edge, unscored tablets debossed with stylized b on one side and 712 on the other side each containing 1 mg of norethindrone acetate and 30 mcg of ethinyl estradiol; nine light blue, round, flat-faced, beveled-edge, unscored tablets debossed with stylized b on one side and 713 on the other side each containing 1 mg of norethindrone acetate and 35 mcg of ethinyl estradiol; and seven brown, round, flat-faced, beveled-edge, unscored tablets debossed with stylized b on one side and 247 on the other side each containing 75 mg ferrous fumarate. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. NDC 0555-9032-70 Carton of 5 Tablet Dispensers Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light. Store tablets inside pouch when not in use. REFERENCES AVAILABLE UPON REQUEST . Teva Pharmaceuticals USA, Inc. North Wales, PA 19454 Rev. G 3/2023
Indications & Usage
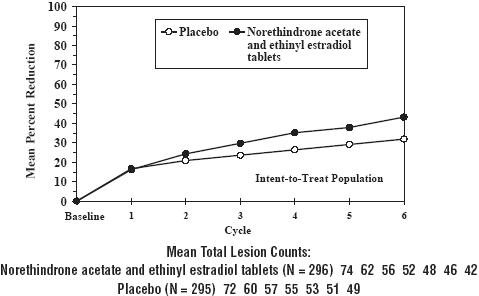
INDICATIONS AND USAGE Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) is indicated for the prevention of pregnancy in women who elect to use combined oral contraceptives as a method of contraception. Tri-Legest ® Fe is indicated for the treatment of moderate acne vulgaris in females, ≥15 years of age, who have no known contraindications to combined oral contraceptive therapy, desire oral contraception, have achieved menarche, and are unresponsive to topical anti-acne medications. Tri-Legest ® Fe should be used for the treatment of acne only if the patient desires a combined oral contraceptive for birth control and plans to stay on it for at least 6 months. Combined oral contraceptives are highly effective for pregnancy prevention. Table 2 lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates. Table 2. Percentage of Women Experiencing an Unintended Pregnancy During the First Year of Typical Use and the First Year of Perfect Use of Contraception and the Percentage Continuing Use at the End of the First Year. United States. % of Women Experiencing an Unintended Pregnancy within the First Year of Use % of Women Continuing Use at One Year ‡ Method Typical Use * Perfect Use † (1) (2) (3) (4) Chance § 85 85 Spermicides ¶ 26 6 40 Periodic Abstinence 25 63 Calendar 9 Ovulation Method 3 Symptothermal #Þ 2 Post-ovulation 1 Cap ß Parous Women 40 26 42 Nulliparous Women 20 9 56 Sponge Parous Women 40 20 42 Nulliparous Women 20 9 56 Diaphragm ß 20 6 56 Withdrawal 19 4 Condom à Female (Reality) 21 5 56 Male 14 3 61 Pill 5 71 Progestin only 0.5 Combined 0.1 IUD Progesterone T 2.0 1.5 81 Copper T380A 0.8 0.6 78 LNG 20 0.1 0.1 81 Depo-Provera ® 0.3 0.3 70 Norplant ® and Norplant-2 ® 0.05 0.05 88 Female Sterilization 0.5 0.5 100 Male Sterilization 0.15 0.10 100 Emergency Contraceptives Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75% è . Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception. ð Source: Trussell J, The Essentials of Contraception. In Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Kowel D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York NY: Irvington Publishers, 1998. ‡ Among couples attempting to avoid pregnancy, the percentage who continue to use a method for 1 year. * Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. † Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. § The percentages becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percent who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether. ¶ Foams, creams, gels, vaginal suppositories, and vaginal film. #Þ Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases. ß With spermicidal cream or jelly. à Without spermicides. è The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. The Food and Drug Administration has declared the following brands of combined oral contraceptives to be safe and effective for emergency contraception: Ovral ® (1 dose is 2 white pills), Alesse ® (1 dose is 5 pink pills), Nordette ® or Levlen ® (1 dose is 4 light-orange pills), Lo/Ovral ® (1 dose is 4 white pills), Triphasil ® or Tri-Levlen ® (1 dose is 4 yellow pills). ð However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches 6 months of age. Norethindrone acetate and ethinyl estradiol tablets were evaluated for the treatment of acne vulgaris in two randomized, double-blind, placebo-controlled, multicenter, Phase 3, six (28-day) cycle studies. A total of 296 patients received norethindrone acetate and ethinyl estradiol tablets and 295 received placebo. Mean age at enrollment for both groups was 24 years. At six months each study demonstrated a statistically significant difference between norethindrone acetate and ethinyl estradiol tablets and placebo for mean change from baseline in lesion counts (see Table 3 and Figure 2). Each study also demonstrated overall treatment success in the investigator’s global evaluation. Patients with severe androgen excess were not studied. Table 3. Acne Vulgaris Indication Pooled Data 376 to 403 and 376 to 404 Observed Means at Six Months and at Baseline * Intent To Treat Population Norethindrone Acetate and Ethinyl Estradiol Tablets N = 296 Placebo N = 295 Difference in Counts Between Norethindrone Acetate and Ethinyl Estradiol Tablets and Placebo at Six Months (95% CI) † Number of Lesions Counts % reduction Counts % reduction INFLAMMATORY LESIONS Baseline Mean 29 29 Six Month Mean 14 52% 17 41% 3 (±2) NON-INFLAMMATORY LESIONS Baseline Mean 44 43 Six Month Mean 27 38% 32 25% 5 (±3.5) TOTAL LESIONS Baseline Mean 74 72 Six Month Mean 42 43% 49 32% 7 (±5) * Numbers rounded to nearest integer † Limits for 95% Confidence Interval; not adjusted for baseline differences Norethindrone acetate and ethinyl estradiol tablets users who started with about 74 acne lesions had about 42 lesions after 6 months of treatment. Placebo users who started with about 72 acne lesions had about 49 lesions after the same duration of treatment. Figure 2. Mean Percent Reduction in Total Lesion Counts From Baseline to Each 28-Day Cycle and Mean Total Lesion Counts at Each Cycle Following Administration of Norethindrone acetate and ethinyl estradiol tablets and Placebo (Statistically significant differences were not found in both studies individually until cycle 6) Figure 2
Dosage and Administration
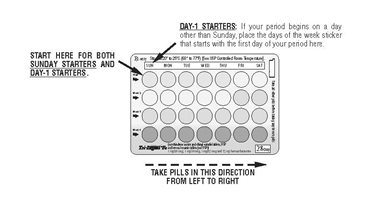
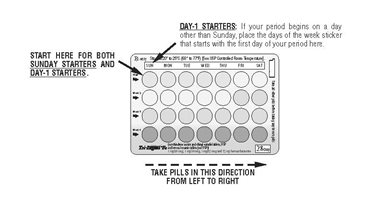
DOSAGE AND ADMINISTRATION The tablet dispenser has been designed to make Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) dosing as easy and as convenient as possible. The tablets are arranged in four rows of seven tablets each, with the days of the week appearing on the tablet dispenser above the first row of tablets. Note: Each tablet dispenser has been preprinted with the days of the week, starting with Sunday, to facilitate a Sunday-Start regimen. Six different days of the week stickers have been provided with the Detailed Patient & Brief Summary Patient Package Insert in order to accommodate a Day-1 Start regimen. If the patient is using the Day-1 Start regimen, she should place the self-adhesive days of the week sticker that corresponds to her starting day over the preprinted days. Important: The patient should be instructed to use an additional method of protection until after the first week of administration in the initial cycle when utilizing the Sunday-Start regimen. The possibility of ovulation and conception prior to initiation of use should be considered. Dosage and Administration for 28-Day Dosage Regimen To achieve maximum contraceptive effectiveness, Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) should be taken exactly as directed and at intervals not exceeding 24 hours. Tri-Legest ® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets [not USP]) provides a continuous administration regimen consisting of 21 light pink, light yellow, and light blue tablets of norethindrone acetate and ethinyl estradiol and seven brown non-hormone containing tablets of ferrous fumarate. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen and do not serve any therapeutic purpose. There is no need for the patient to count days between cycles because there are no “off-tablet days”. A. Sunday-Start Regimen: The patient begins taking the first light pink tablet from the top row of the tablet dispenser (labeled Sunday) on the first Sunday after menstrual flow begins. When menstrual flow begins on Sunday, the first light pink tablet is taken on the same day. The patient takes one tablet daily for 21 days. The last light blue tablet in the dispenser will be taken on a Saturday. Upon completion of all 21 tablets, and without interruption, the patient takes one brown tablet daily for 7 days. Upon completion of this first course of tablets, the patient begins a second course of 28-day tablets, without interruption, the next day (Sunday), starting with the Sunday light pink tablet in the top row. Adhering to this regimen of one tablet daily for 21 days, followed without interruption by one brown tablet daily for 7 days, the patient will start all subsequent cycles on a Sunday. B. Day-1 Start Regimen: The first day of menstrual flow is Day 1. The patient places the self-adhesive days of the week sticker that corresponds to her starting day over the preprinted days on the tablet dispenser. She starts taking one light pink tablet daily, beginning with the first light pink tablet in the top row. After the last light blue tablet (at the end of the third row) has been taken, the patient will then take the brown tablets for a week (7 days). For all subsequent cycles, the patient begins a new 28 tablet regimen on the eighth day after taking her last light blue tablet, again starting with the first tablet in the top row after placing the appropriate days of the week sticker over the preprinted days on the tablet dispenser. Following this regimen of 21 light pink, light yellow, and light blue tablets and 7 brown tablets, the patient will start all subsequent cycles on the same day of the week as the first course. Tablets should be taken regularly at the same time each day and can be taken without regard to meals. It should be stressed that efficacy of medication depends on strict adherence to the dosage schedule. Special Notes on Administration Menstruation usually begins two or three days, but may begin as late as the fourth or fifth day, after the brown tablets have been started. In any event, the next course of tablets should be started without interruption. If spotting occurs while the patient is taking light pink, light yellow, or light blue tablets, continue medication without interruption. If the patient forgets to take one or more light pink , light yellow or light blue tablets, the following is suggested: One tablet is missed take tablet as soon as remembered take next tablet at the regular time Two consecutive tablets are missed (Week 1 or Week 2) take two tablets as soon as remembered take two tablets the next day use another birth control method for seven days following the missed tablets Two consecutive tablets are missed (Week 3) Sunday-Start Regimen: take one tablet daily until Sunday discard remaining tablets start new pack of tablets immediately (Sunday) use another birth control method for seven days following the missed tablets Day-1 Start Regimen: discard remaining tablets start new pack of tablets that same day use another birth control method for seven days following the missed tablets Three (or more) consecutive tablets are missed Sunday-Start Regimen: take one tablet daily until Sunday discard remaining tablets start new pack of tablets immediately (Sunday) use another birth control method for seven days following the missed tablets Day-1 Start Regimen: discard remaining tablets start new pack of tablets that same day use another birth control method for seven days following the missed tablets The possibility of ovulation occurring increases with each successive day that scheduled light pink, light yellow and light blue tablets are missed. While there is little likelihood of ovulation occurring if only one tablet is missed, the possibility of spotting or bleeding is increased. This is particularly likely to occur if two or more consecutive light pink, light yellow, and light blue tablets are missed. If the patient forgets to take any of the seven brown tablets in week four, those brown tablets that were missed are discarded and one brown tablet is taken each day until the pack is empty. A back-up birth control method is not required during this time. A new pack of tablets should be started no later than the eighth day after the last light blue tablet was taken. In the rare case of bleeding which resembles menstruation, the patient should be advised to discontinue medication and then begin taking tablets from a new tablet dispenser on the next Sunday or the first day (Day 1) depending on her regimen. Persistent bleeding which is not controlled by this method indicates the need for reexamination of the patient, at which time nonfunctional causes should be considered. Use of Combined Oral Contraceptives in the Event of a Missed Menstrual Period 1. If the patient has not adhered to the prescribed dosage regimen, the possibility of pregnancy should be considered after the first missed period and combined oral contraceptives should be withheld until pregnancy has been ruled out. 2. If the patient has adhered to the prescribed regimen and misses two consecutive periods, pregnancy should be ruled out before continuing the contraceptive regimen. After several months on treatment, bleeding may be reduced to a point of virtual absence. This reduced flow may occur as a result of medication, in which event it is not indicative of pregnancy. Acne The timing of initiation of dosing with Tri-Legest ® Fe for acne should follow the guidelines for use of Tri-Legest ® Fe as a combined oral contraceptive. Consult the DOSAGE AND ADMINISTRATION section for Tri-Legest ® Fe oral contraceptives.
