Drug Catalog - Product Detail
OXYBUTYNIN CHLORIDE ER 5MG TB 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 27241-0155-08 | AJANTA PHARMA LIMITED | 500 | 5MG | TABLET |
PACKAGE FILES




Generic Name
OXYBUTYNIN CHLORIDE
Substance Name
OXYBUTYNIN CHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA211655
Description
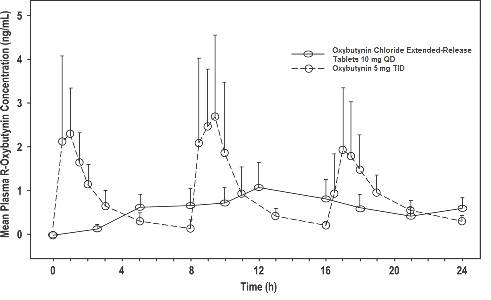
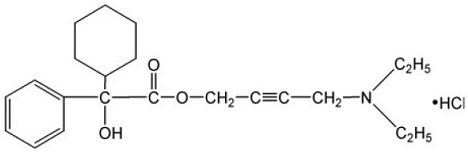
11 DESCRIPTION Oxybutynin chloride, USP is an antispasmodic, muscarinic antagonist. Each oxybutynin chloride extended-release tablet, USP contains 5 mg, 10 mg, or 15 mg of oxybutynin chloride USP, formulated as a once-a-day controlled-release tablet for oral administration. Oxybutynin chloride, USP is administered as a racemate of R- and S-enantiomers. Chemically, oxybutynin chloride, USP is 4(diethylamino-but-2-ynyl (RS) 2-cyclohexyl-2-hydroxy 2 phenyl acetate hydrochloride. The molecular formula of oxybutynin chloride, USP is C 22 H 31 NO 3 •HCl. Its structural formula is: Oxybutynin chloride, USP is a white crystalline powder with a molecular weight of 393.95. It is freely soluble in water and in alcohol, very soluble in methanol and in chloroform, soluble in acetone, slightly soluble in ether, very slightly soluble in hexane. Oxybutynin chloride extended-release tablets, USP also contains the following inert ingredients: anhydrous dibasic calcium phosphate, povidone, hypromellose, colloidal silicon dioxide, magnesium stearate, polyethylene glycol, methacrylic acid copolymer type c, talc, titanium dioxide, triethyl citrate, sodium bicarbonate, sodium lauryl sulfate, ferrosoferric oxide, ferric oxide red (in 10 mg only) and iron oxide yellow (in 5 mg and 15 mg only). The imprinting material contains: shellac glaze, black iron oxide, propylene glycol and ammonium hydroxide. Meets USP Dissolution Test 9. System Components and Performance Oxybutynin chloride extended-release tablets, USP are formulated to deliver oxybutynin chloride at a controlled rate over approximately 24 hours. The dosage form is comprised of a hydrophilic cellulose polymer matrix tablet surrounded by an enteric coating system. The enteric coat is insoluble in the low pH environment of the stomach. As the tablet passes through the stomach and enters the higher pH environment of the small intestine, the enteric coating dissolves and/or erodes to expose the polymer matrix tablet which swells and releases drug at a controlled rate via diffusion and/or erosion. oxybutynin-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Oxybutynin chloride extended-release tablets, USP are available in three dosage strengths, 5 mg (light yellow to yellow), 10 mg (light pink to pink), and 15 mg (light grey to grey) and are imprinted on one side with "0B1", "0B2", or "0B3" with black ink. Oxybutynin chloride extended-release tablets, USP are supplied in bottles of 100 tablets and 500 tablets. 5 mg 100 count bottle with child resistant closures NDC 27241-155-04 500 count bottle NDC 27241-155-08 10 mg 100 count bottle with child resistant closures NDC 27241-156-04 500 count bottle NDC 27241-156-08 15 mg 100 count bottle with child resistant closures NDC 27241-157-04 500 count bottle NDC 27241-157-08 Storage Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [see USP Controlled Room Temperature]. Protect from moisture and humidity. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Oxybutynin chloride is a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. Oxybutynin chloride extended-release tablets is also indicated for the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida). Oxybutynin chloride is a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. ( 1 ) Oxybutynin chloride extended-release tablets are also indicated for the treatment of pediatric patients aged 6 years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g., spina bifida). ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be administered with or without food. Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be administered with or without food. ( 2 ) Adults: Start with 5 mg or 10 mg, once daily at approximately the same time every day. Dose should not exceed 30 mg per day. ( 2.1 ) Pediatric patients (6 years of age or older): Start with 5 mg, once daily at approximately the same time every day. Dose should not exceed 20 mg per day. ( 2.2 ) 2.1 Adults The recommended starting dose of oxybutynin chloride extended-release tablets is 5 mg or 10 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 30 mg/day). In general, dosage adjustment may proceed at approximately weekly intervals. 2.2 Pediatric Patients Aged 6 Years of Age and Older The recommended starting dose of oxybutynin chloride extended-release tablets is 5 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 20 mg/day).
