Drug Catalog - Product Detail
Paliperidone Extended Release 1.5mg Tablet 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0280-03 | AMNEAL PHARMACEUTICALS | 30 | 1.5MG | TABLET |
PACKAGE FILES



Generic Name
PALIPERIDONE
Substance Name
PALIPERIDONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204707
Description
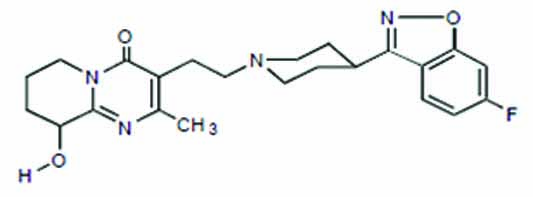
11 DESCRIPTION Paliperidone extended-release tablets contains paliperidone USP, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. Paliperidone, USP contains a racemic mixture of (+)- and (-)- paliperidone. The chemical name is (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one. Its molecular formula is C 23 H 27 FN 4 O 3 and its molecular weight is 426.49. The structural formula is: Paliperidone, USP is sparingly soluble in 0.1N HCl and methylene chloride; practically insoluble in water, 0.1N NaOH, and hexane; and slightly soluble in N,N-dimethylformamide. Paliperidone extended-release tablets are intended for oral administration and are available in 1.5 mg (brown), 3 mg (white), 6 mg (light beige), and 9 mg (pink) strengths. Inactive ingredients are colloidal silicon dioxide, fumaric acid, hypromellose, lactose monohydrate, macrogol, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, polyethylene oxides, povidone, talc and triethyl citrate. The 1.5 mg tablets also contain iron oxide black, iron oxide red and iron oxide yellow. The 6 mg tablets also contain iron oxide red and iron oxide yellow. The 9 mg tablets also contain iron oxide black, iron oxide red and iron oxide yellow. chem structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Paliperidone extended-release tablets, 1.5 mg , are supplied as brown, round, biconvex tablets, debossed “AN” over “80” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-280-03 Bottles of 90: NDC 65162-280-09 Paliperidone extended-release tablets, 3 mg , are supplied as white, round, biconvex tablets, debossed “AN” over “81” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-281-03 Bottles of 90: NDC 65162-281-09 Paliperidone extended-release tablets, 6 mg , are supplied as light beige, round, biconvex tablets, debossed “AN” over “82” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-282-03 Bottles of 90: NDC 65162-282-09 Paliperidone extended-release tablets, 9 mg , are supplied as pink, round, biconvex tablets, debossed “AN” over “83” on one side and plain on the other side. They are available as follows: Bottles of 30: NDC 65162-283-03 Bottles of 90: NDC 65162-283-09 Storage and Handling Store at 20° to 25ºC (68° to 77ºF); excursions permitted between 15° to 30ºC (59° to 86ºF) [see USP Controlled Room Temperature]. Protect from moisture. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Paliperidone extended-release tablets are an atypical antipsychotic agent indicated for Treatment of schizophrenia (1.1) Adults: Efficacy was established in three 6-week trials and one maintenance trial. (14.1) Adolescents (ages 12 to 17): Efficacy was established in one 6-week trial. (14.1) Treatment of schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers and/or antidepressants. (1.2) Efficacy was established in two 6-week trials in adult patients. (14.2) 1.1 Schizophrenia Paliperidone extended-release tablets are indicated for the treatment of schizophrenia [see Clinical Studies (14.1) ]. The efficacy of paliperidone extended-release tablets in schizophrenia was established in three 6-week trials in adults and one 6-week trial in adolescents, as well as one maintenance trial in adults. 1.2 Schizoaffective Disorder Paliperidone extended-release tablets are indicated for the treatment of schizoaffective disorder as monotherapy and an adjunct to mood stabilizers and/or antidepressant therapy [see Clinical Studies (14.2) ] . The efficacy of paliperidone in schizoaffective disorder was established in two 6-week trials in adults.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Initial Dose Recommended Dose Maximum Dose Schizophrenia - adults (2.1) 6 mg/day 3 to 12 mg/day 12 mg/day Schizophrenia-adolescents (2.1) Weight < 51 kg 3 mg/day 3 to 6 mg/day 6 mg/day Weight ≥ 51 kg 3 mg/day 3 to 12 mg/day 12 mg/day Schizoaffective disorder - adults (2.2) 6 mg/day 3 to 12 mg/day 12 mg/day Tablet should be swallowed whole and should not be chewed, divided, or crushed. (2.3) 2.1 Schizophrenia Adults The recommended dose of paliperidone extended-release tablets for the treatment of schizophrenia in adults is 6 mg administered once daily. Initial dose titration is not required. Although it has not been systematically established that doses above 6 mg have additional benefit, there was a general trend for greater effects with higher doses. This must be weighed against the dose-related increase in adverse reactions. Thus, some patients may benefit from higher doses, up to 12 mg/day, and for some patients, a lower dose of 3 mg/day may be sufficient. Dose increases above 6 mg/day should be made only after clinical reassessment and generally should occur at intervals of more than 5 days. When dose increases are indicated, increments of 3 mg/day are recommended. The maximum recommended dose is 12 mg/day. In a longer-term study, paliperidone extended-release tablets have been shown to be effective in delaying time to relapse in patients with schizophrenia who were stabilized on paliperidone extended-release tablets for 6 weeks [see Clinical Studies (14) ]. Paliperidone extended-release tablets should be prescribed at the lowest effective dose for maintaining clinical stability and the physician should periodically reevaluate the long-term usefulness of the drug in individual patients. Adolescents (12 to 17 years of age) The recommended starting dose of paliperidone extended-release tablets for the treatment of schizophrenia in adolescents 12 to 17 years of age is 3 mg administered once daily. Initial dose titration is not required. Dose increases, if considered necessary, should be made only after clinical reassessment and should occur at increments of 3 mg/day at intervals of more than 5 days. Prescribers should be mindful that, in the adolescent schizophrenia study, there was no clear enhancement to efficacy at the higher doses, i.e., 6 mg for subjects weighing less than 51 kg and 12 mg for subjects weighing 51 kg or greater, while adverse events were dose-related. 2.2 Schizoaffective Disorder The recommended dose of paliperidone extended-release tablets for the treatment of schizoaffective disorder in adults is 6 mg administered once daily. Initial dose titration is not required. Some patients may benefit from lower or higher doses within the recommended dose range of 3 to 12 mg once daily. A general trend for greater effects was seen with higher doses. This trend must be weighed against dose-related increase in adverse reactions. Dosage adjustment, if indicated, should occur only after clinical reassessment. Dose increases, if indicated, generally should occur at intervals of more than 4 days. When dose increases are indicated, increments of 3 mg/day are recommended. The maximum recommended dose is 12 mg/day. 2.3 Administration Instructions Paliperidone extended-release tablets can be taken with or without food. Paliperidone extended-release tablets must be swallowed whole with the aid of liquids. Tablets should not be chewed, divided, or crushed. The medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell, along with insoluble core components, is eliminated from the body; patients should not be concerned if they occasionally notice in their stool something that looks like a tablet. 2.4 Use with Risperidone Concomitant use of paliperidone extended-release tablets with risperidone has not been studied. Since paliperidone is the major active metabolite of risperidone, consideration should be given to the additive paliperidone exposure if risperidone is co-administered with paliperidone extended-release tablets. 2.5 Dosage in Special Populations Renal Impairment Dosing must be individualized according to the patient’s renal function status. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min), the recommended initial dose of paliperidone extended-release tablets is 3 mg once daily. The dose may then be increased to a maximum of 6 mg once daily based on clinical response and tolerability. For patients with moderate to severe renal impairment (creatinine clearance ≥ 10 mL/min to < 50 mL/min), the recommended initial dose of paliperidone extended-release tablets is 1.5 mg once daily, which may be increased to a maximum of 3 mg once daily after clinical reassessment. As paliperidone extended-release tablets have not been studied in patients with creatinine clearance below 10 mL/min, use is not recommended in such patients [see Clinical Pharmacology (12.3) ] . Hepatic Impairment For patients with mild to moderate hepatic impairment, (Child-Pugh Classification A and B), no dose adjustment is recommended [see Clinical Pharmacology (12.3) ]. Paliperidone extended-release tablets have not been studied in patients with severe hepatic impairment. Elderly Because elderly patients may have diminished renal function, dose adjustments may be required according to their renal function status. In general, recommended dosing for elderly patients with normal renal function is the same as for younger adult patients with normal renal function. For patients with moderate to severe renal impairment (creatinine clearance 10 mL/min to < 50 mL/min), the maximum recommended dose of paliperidone extended-release tablets are 3 mg once daily [see Renal Impairment above].
