Drug Catalog - Product Detail
PAROXETINE HCL ER TB 37.5MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62175-0472-32 | KREMERS URBAN | 30 | 37.5MG | TABLET |
PACKAGE FILES





Generic Name
PAROXETINE HYDROCHLORIDE
Substance Name
PAROXETINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204744
Description
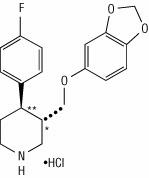
11 DESCRIPTION Paroxetine Extended-Release Tablets, USP contain paroxetine hydrochloride, an SSRI. It is the hydrochloride salt of a phenylpiperidine compound identified chemically as (-)- trans -4 R- (4'-fluorophenyl)-3 S -[(3',4'-methylenedioxyphenoxy) methyl] piperidine hydrochloride hemihydrate and has the empirical formula of C 19 H 20 FNO 3 •HCl•1/2H 2 O. The molecular weight is 374.8 g/mol (329.4 g/mol as free base). The structural formula of paroxetine hydrochloride is: Paroxetine hydrochloride is an odorless, off-white powder, having a melting point range of 120° C to 138°C and a solubility of 5.4 mg/mL in water. Paroxetine Extended-Release Tablets, USP are intended for oral administration. Each enteric, film-coated, extended-release tablet contains paroxetine hydrochloride equivalent to paroxetine as follows: 12.5 mg–white, 25 mg–pink and 37.5 mg–blue. Inactive ingredients consist of hypromellose, lactose monohydrate, magnesium stearate, methacrylic acid and ethyl acrylate copolymer dispersion, polyethylene glycols, polyvinyl alcohol, povidone, silicon dioxide, talc, titanium dioxide, triethyl citrate. In addition, the 25 mg and 37.5 mg colorant contains FD&C Blue No. 2 aluminum lake. In addition, the 25 mg colorant also contains carmine. Paroxetine meets USP Dissolution Test 3. Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Paroxetine Extended-Release Tablets, USP are supplied as film-coated, extended-release, round convex tablets, as follows: 12.5 mg white tablets (debossed with “KU” on one side and “470” on the other) NDC 62175-470-32 Bottles of 30 NDC 62175-470-41 Bottles of 500 25 mg pink tablets (debossed with “KU” on one side and “471” on the other) NDC 62175-471-32 Bottles of 30 NDC 62175-471-41 Bottles of 500 37.5 mg blue tablets (debossed with “KU” on one side and “472” on the other) NDC 62175-472-32 Bottles of 30 NDC 62175-472-41 Bottles of 500 Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Paroxetine is indicated in adults for the treatment of: Major depressive disorder (MDD) Panic disorder (PD) Social anxiety disorder (SAD) Premenstrual dysphoric disorder (PMDD) Paroxetine is a selective serotonin reuptake inhibitor (SSRI) indicated in adults for the treatment of ( 1 ): Major Depressive Disorder (MDD) Panic Disorder (PD) Social Anxiety Disorder (SAD) Premenstrual Dysphoric Disorder (PMDD)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Swallow tablet whole; do not chew or crush. ( 2 . 1 ) Recommended starting and maximum daily dosage: ( 2.2 , 2.3 ) Indication Starting Dose Maximum Dose MDD 25 mg/day 62.5 mg/day PD 12.5 mg/day 75 mg/day SAD 12.5 mg/day 37.5 mg/day PMDD 12.5 mg/day 25 mg/day For PMDD, dose continuously or intermittently (luteal phase only). ( 2.3 ) If inadequate response to starting dosage, titrate in 12.5 mg per day increments once weekly. ( 2.2 , 2.3 ) Elderly patients, patients with severe renal impairment or severe hepatic impairment: Starting dose is 12.5 mg per day. Do not exceed 50 mg per day for treatment of MDD and PD and 37.5 mg per day for treatment of SAD. ( 2.5 ) When discontinuing Paroxetine, reduce dose gradually. ( 2.7 ) 2.1 Important Administration Instructions Administer Paroxetine as a single daily dose in the morning, with or without food. Swallow tablets whole and do not chew or crush. 2.2 Dosage in Patients with Major Depressive Disorder, Panic Disorder, and Social Anxiety Disorder The recommended initial dosage and maximum dosage of Paroxetine in patients with MDD, PD, and SAD are presented in Table 1. In patients with an inadequate response, dosage may be increased in increments of 12.5 mg per day at intervals of at least 1 week, depending on tolerability. Table 1: Recommended Daily Dosage of Paroxetine in Patients with MDD, PD, and SAD Indication Starting Dose Maximum Dose MDD 25 mg 62.5 mg PD 12.5 mg 75 mg SAD 12.5 mg 37.5 mg 2.3 Dosage in Patients with Premenstrual Dysphoric Disorder The recommended starting dosage in women with PMDD is 12.5 mg per day. Paroxetine may be administered either continuously (every day throughout the menstrual cycle) or intermittently (only during the luteal phase of the menstrual cycle, i.e., starting the daily dosage 14 days prior to the anticipated onset of menstruation and continuing through the onset of menses). Intermittent dosing is repeated with each new cycle. In patients with an inadequate response, the dosage may be increased to the maximum recommended dosage of 25 mg per day, depending on tolerability. Institute dosage adjustments at intervals of at least 1 week. 2.4 Screen for Bipolar Disorder Prior to Starting Paroxetine Prior to initiating treatment with Paroxetine or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions ( 5.6 )] . 2.5 Dosage Modifications for Elderly Patients, Patients with Severe Renal Impairment and Patients with Severe Hepatic Impairment The recommended initial dose of Paroxetine is 12.5 mg per day for elderly patients, patients with severe renal impairment, and patients with severe hepatic impairment. Reduce initial dose and increase up-titration intervals if necessary. Dosage should not exceed 50 mg per day for MDD or PD and should not exceed 37.5 mg per day for SAD [see Use in Specific Populations ( 8.5 , 8.6 )] . 2.6 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of Paroxetine. In addition, at least 14 days must elapse after stopping Paroxetine before starting an MAOI antidepressant [see Contraindications ( 4 ), Warnings and Precautions ( 5.2 )] . 2.7 Discontinuation of Treatment with Paroxetine Adverse reactions may occur upon discontinuation of Paroxetine [see Warnings and Precautions ( 5.7 )] . Gradually reduce the dosage rather than stopping Paroxetine abruptly whenever possible.
