Drug Catalog - Product Detail
PENICILLIN G POT. FOR INJECTION INJECT. 5MM/ML 10X30ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00781-6135-95 | SANDOZ | 1 | 5000000UNIT | NA |
PACKAGE FILES


Generic Name
PENICILLIN G POTASSIUM
Substance Name
PENICILLIN G POTASSIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAMUSCULAR
Application Number
ANDA065079
Description
DESCRIPTION Buffered penicillin G potassium for injection, USP is sterile penicillin G potassium powder for reconstitution. It is an antibacterial agent intended for intravenous or intramuscularly use. Chemically, penicillin G potassium is monopotassium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate, and has the following chemical structure: Molecular Formula: C 16 H 17 KN 2 O 4 S Molecular Weight: 372.48 Penicillin G potassium, a water soluble benzylpenicillin, is a white to almost white crystalline powder which is almost odorless and/or after reconstitution a colorless solution. The pH of freshly constituted solutions usually ranges from 6 to 8.5. Sodium citrate and citric acid have been added as a buffer. Buffered penicillin G potassium for injection, USP is supplied in vials equivalent to 1,000,000 units (1 million units), 5,000,000 units (5 million units), or 20,000,000 units (20 million units) of penicillin G as the potassium salt. Each million unit contains approximately 7.9 milligrams of sodium (0.34 mEq) and 65.6 milligrams of potassium (1.68 mEq). chemical-structure
How Supplied
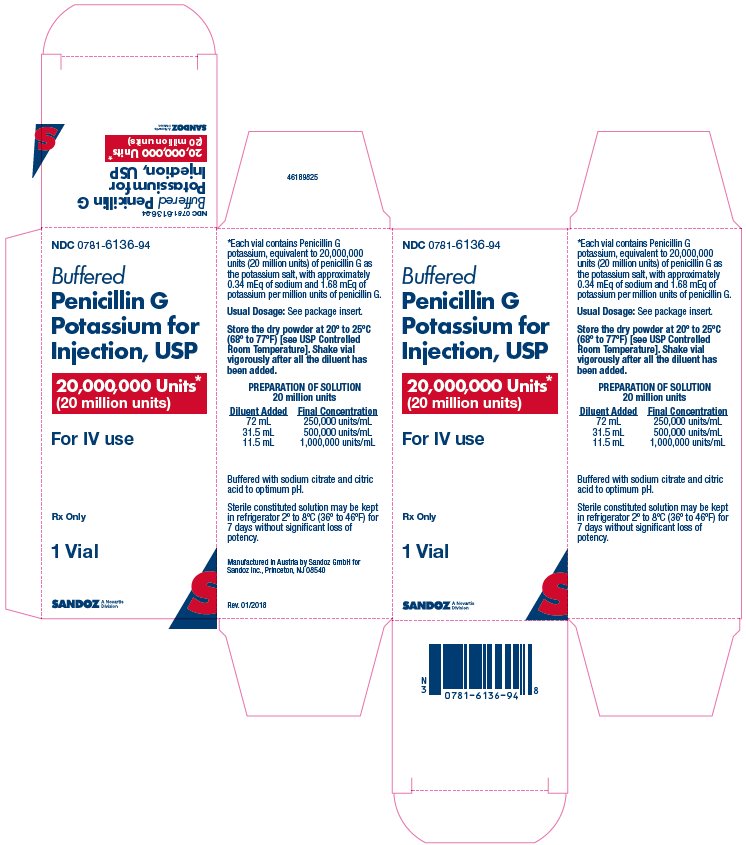
HOW SUPPLIED Buffered penicillin G potassium for injection, USP, is supplied in dry powder form in vials containing: 1,000,000 units (1 million units) × 10’s (NDC 0781-6134-95) 5,000,000 units (5 million units) × 10’s (NDC 0781-6135-95) 20,000,000 units (20 million units) × 1’s (NDC 0781-6136-94) of crystalline penicillin G as the potassium salt; buffered with sodium citrate and citric acid to an optimum pH. Storage Store the dry powder at 20° to 25°C (68° to 77°F) [see USP controlled room temperature]. Sterile constituted solution may be kept in refrigerator 2° to 8°C (36° to 46°F) for 7 days without significant loss of potency.
Indications & Usage
INDICATIONS AND USAGE Therapy Buffered penicillin G potassium for injection is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below. Appropriate culture and susceptibility tests should be done before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to penicillin G. Therapy with Buffered penicillin G potassium for injection may be initiated before results of such tests are known when there is reason to believe the infection may involve any of the organisms listed below, however, once these results become available, appropriate therapy should be continued. CLINICAL INDICATION INFECTING ORGANISM Septicemia, empyema, pneumonia, pericarditis, endocarditis, meningitis Streptococcus pyogenes (group A beta-hemolytic streptococcus ), other beta-hemolytic streptococci including groups C, H, G, L and M, Streptococcus pneumoniae and Staphylococcus species (non-penicillinase producing strains) Anthrax Bacillus anthracis Actinomycosis (cervicofacial disease and thoracic and abdominal disease) Actinomyces Israelil Botulism (adjunctive therapy to antitoxin), gas gangrene, and tetanus (adjunctive therapy to human tetanus immune globulin) Clostridium species Diphtheria (adjunctive therapy to antitoxin and prevention of the carrier state) Corynebacterium diphtheriae Erysipelothrix endocarditis Erysipelothrix rhusiopthiae Fusospirochetosis (severe infections of the oropharynx [Vincent’s], lower respiratory tract and genital area) Fusobacterium species and spirochetes Listeria infections including meningitis and endocarditis Listeria monocytogenes Pasteurella infections including bacteremia and meningitis Pasteurella multocida Haverhill fever Streptobacillus moniliformis Rat-bite fever Spirillum minus or Streptobacillus moniliformis Disseminated gonococcal infections Neisseria gonorrhoeae (penicillin-susceptible) Syphilis (congenital and neurosyphilis) Treponema pallidum Meningococcal meningitis and/or septicemia Neisseria meningitidis Gram-negative bacillary infections (bacteremias) Escherichia coli, Enterobacter aerogenes, Alcaligenes faecalis, salmonella, shigella and Proteus mirabilis, Penicillin G is not the drug of choice in the treatment of gram-negative bacillary infections. To reduce the development of drug-resistant bacteria and maintain the effectiveness of penicillin G potassium and other antibacterial drugs, penicillin G potassium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Buffered penicillin G potassium for injection may be given intravenously or intramuscularly. The usual dose recommendations are as follows: CLINICAL INDICATION DOSAGE Serious infections due to susceptible strains of streptococci (including S. pneumoniae) and staphylococci-septicemia , empyema, pneumonia, pericarditis, endocarditis and meningitis 5 to 24 million units/day depending on the infection and its severity administered in equally divided doses every 4 to 6 hours Anthrax Minimum of 8 million units/day in divided doses every 6 hours. Higher doses may be required depending on susceptibility of organism. Actinomycosis Cervicofacial disease 1 to 6 million units/day Thoracic and abdominal disease 10 to 20 million units/day Clostridial infections Botulism (adjunctive therapy to antitoxin) 20 million units/day Gas gangrene (debridement and/or surgery as indicated) Tetanus (adjunctive therapy to human tetanus immune globulin) Diphtheria (adjunctive therapy to antitoxin and for the prevention of the carrier state) 2 to 3 million units/day in divided doses for 10 to 12 days Erysipelothrix endocarditis 12 to 20 million units/day for 4 to 6 weeks Fusospirochetosis (severe infections of the oropharnyx [Vincent’s], lower respiratory tract and genital area) 5 to 10 million units/day Listeria infections Meningitis 15 to 20 million units/day for 2 weeks Endocarditis 15 to 20 million units/day for 4 weeks Pasteurella infections including bacteremia and meningitis 4 to 6 million units/day for 2 weeks Haverhill fever, Rat-bite fever 12 to 20 million units/day for 3 to 4 weeks Disseminated gonococcal infections, such as meningitis endocarditis, arthritis, etc., caused by penicillin-susceptible organisms 10 million units/day; duration depends on the type of infection Syphilis (neurosyphilis) 12 to 24 million units/day, as 2 to 4 MU every 4 hours for 10 to 14 days; many experts recommend additional therapy with Benzathine PCN G 2.4 MU intramuscular weekly for 3 doses after completion of intravenous therapy Meningococcal meningitis and/or septicemia 24 million units/day as 2 million units every 2 hours * Because of its short half-life, penicillin G is administered in divided doses, usually every 4 to 6 hours with the exception of meningococcal meningitis/septicemia, i.e., every 2 hours. Pediatric patients This product should not be administered to patients requiring less than one million units per dose (see PRECAUTIONS – Pediatric Use ). CLINICAL INDICATION DOSAGE Serious infections, such as pneumonia and endocarditis, due to susceptible strains of streptococci (including S. pneumoniae) and meningococcus 150,000 units/kg/day divided in equal doses every 4 to 6 hours; duration depends on infecting organism and type of infection. Meningitis caused by susceptible strains of pneumococcus and meningococcus 250,000 units/kg/day divided in equal doses every 4 hours for 7 to 14 days depending on the infecting organism (maximum dose of 12 to 20 million units/day) Disseminated Gonococcal infections (penicillin-susceptible strains) weight less than 45 kg: Arthritis 100,000 units/kg/day in 4 equally divided doses for 7 to 10 days Meningitis 250,000 units/kg/day in equal doses every 4 hours for 10 to 14 days Endocarditis 250,000 units/kg/day in equal doses every 4 hours for 4 weeks Arthritis, meningitis, endocarditis weight 45 kg or greater: 10 million units/day in 4 equally divided doses with the duration of therapy depending on the type of infection Syphilis (congenital and neurosyphilis) after the newborn period 200,000 to 300,000 units/kg/day (administered as 50,000 units/kg every 4 to 6 hours) for 10 to 14 days Diphtheria (adjunctive therapy to antitoxin and for prevention of the carrier state) 150,000 to 250,000 units/kg/day in equal doses every 6 hours for 7 to 10 days Rat-bite fever; Haverhill fever (with endocarditis caused by S. moniliformis) 150,000 to 250,000 units/kg/day in equal doses every 4 hours for 4 weeks Renal Impairment Penicillin G is relatively nontoxic, and dosage adjustments are generally required only in cases of severe renal impairment. The recommended dosage regimens are as follows: Creatinine clearance less than 10 mL/min/1.73m 2 ; administer a full loading dose (see recommended dosages in the tables above ) followed by one-half of the loading dose every 8 to 10 hours. Uremic patients with a creatinine clearance greater than 10 mL/min/1.73m 2 ; administer a full loading dose (see recommended dosages in the tables above ) followed by one-half of the loading dose every 4 to 5 hours. Additional dosage modifications should be made in patients with hepatic disease and renal impairment. For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for Group A beta-hemolytic streptococcal infections should be maintained for at least 10 days to reduce the risk of rheumatic fever. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Preparation of Solution Solutions of penicillin should be prepared as follows: Loosen powder. Hold vial horizontally and rotate it while slowly directing the stream of diluent against the wall of the vial. Shake vial vigorously after all the diluent has been added. Depending on the route of administration, use Sterile Water for Injection, USP or Sterile Isotonic Sodium Chloride Solution for Parenteral use. Note: Penicillins are rapidly inactivated in the presence of carbohydrate solutions at alkaline pH. Reconstitution The following table shows the amount of solvent required for solution of various concentrations: Approx. Desired Concentration (units/mL) Approx. Volume for 1,000,000 units (mL) Solvent for Vial of 5,000,000 units (mL) Infusion Only 20,000,000 units (mL) 100,000 9.8 - - 250,000 3.8 18 72 500,000 1.8 8 31.5 750,000 - 4.7 - 1,000,000 - 3 11.5 When the required volume of solvent is greater than the capacity of the vial, the penicillin can be dissolved by first injecting only a portion of the solvent into the vial, then withdrawing the resultant solution and combining it with the remainder of the solvent in a larger sterile container. Penicillin G potassium for injection is highly water soluble. It may be dissolved in small amounts of Water for Injection, or Sterile Isotonic Sodium Chloride Solution for Parenteral Use. All solutions should be stored in a refrigerator. When refrigerated, penicillin solutions may be stored for seven days without significant loss of potency. Buffered penicillin G potassium for injection may be given intramuscularly or by continuous intravenous drip for dosages of 500,000, 1,000,000 or 5,000,000 units. It is also suitable for intrapleural, intraarticular, and other local installations. THE 20,000,000 UNIT (20 MILLION UNIT) DOSAGE MAY BE ADMINISTERED BY INTRAVENOUS INFUSION ONLY. (1) Intramuscular Injection: Keep total volume of injection small. The intramuscular route is the preferred route of administration. Solutions containing up to 100,000 units of penicillin per mL of diluent may be used with a minimum of discomfort. Greater concentration of penicillin G per mL is physically possible and may be employed where therapy demands. When large doses are required, it may be advisable to administer aqueous solutions of penicillin by means of continuous intravenous drip. (2) Continuous Intravenous Drip: Determine the volume of fluid and rate of its administration required by the patient in a 24-hour period in the usual manner for fluid therapy, and add the appropriate daily dosage of penicillin to this fluid. For example, if an adult patient requires 2 liters of fluid in 24 hours and a daily dosage of 10 million units of penicillin, add 5 million units of 1 liter and adjust the rate of flow so the liter will be infused in 12 hours. (3) Intrapleural or Other Local Infusion: If fluid is aspirated, give infusion in a volume equal to 1/4 or 1/2 the amount of fluid aspirated, otherwise, prepare as for an intramuscular injection. (4) Intrathecal Use: The intrathecal use of penicillin in meningitis must be highly individualized. It should be employed only with full consideration of the possible irritating effects of penicillin when used by this route. The preferred route of therapy in bacterial meningitides is intravenous, supplemented by intramuscular injection.
