Drug Catalog - Product Detail
POTASSIUM CHLORIDE ER 8MEQ TB 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0986-01 | AUROBINDO PHARMA | 100 | 8MEQ | NA |
PACKAGE FILES

Generic Name
POTASSIUM CHLORIDE
Substance Name
POTASSIUM CHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210921
Description
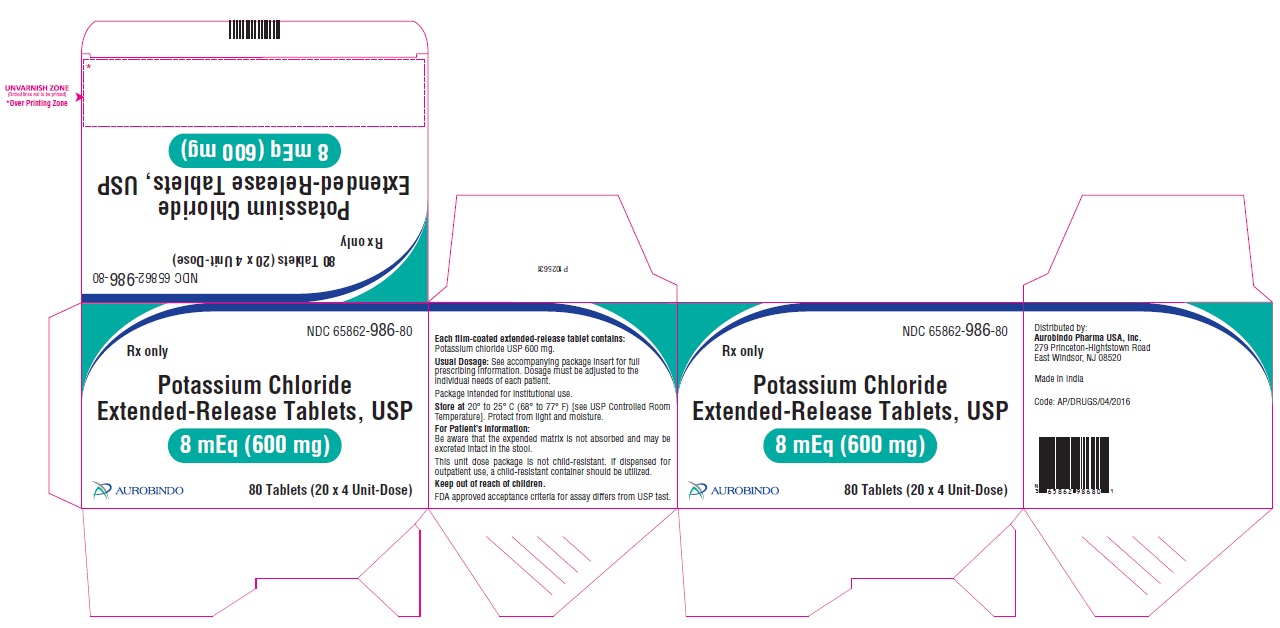
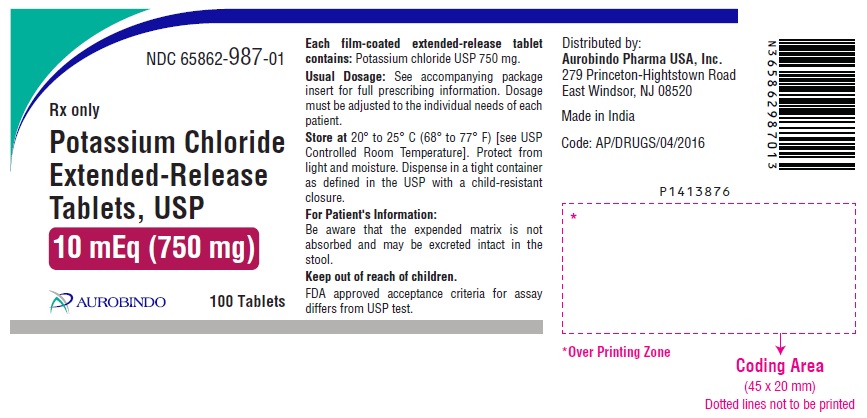
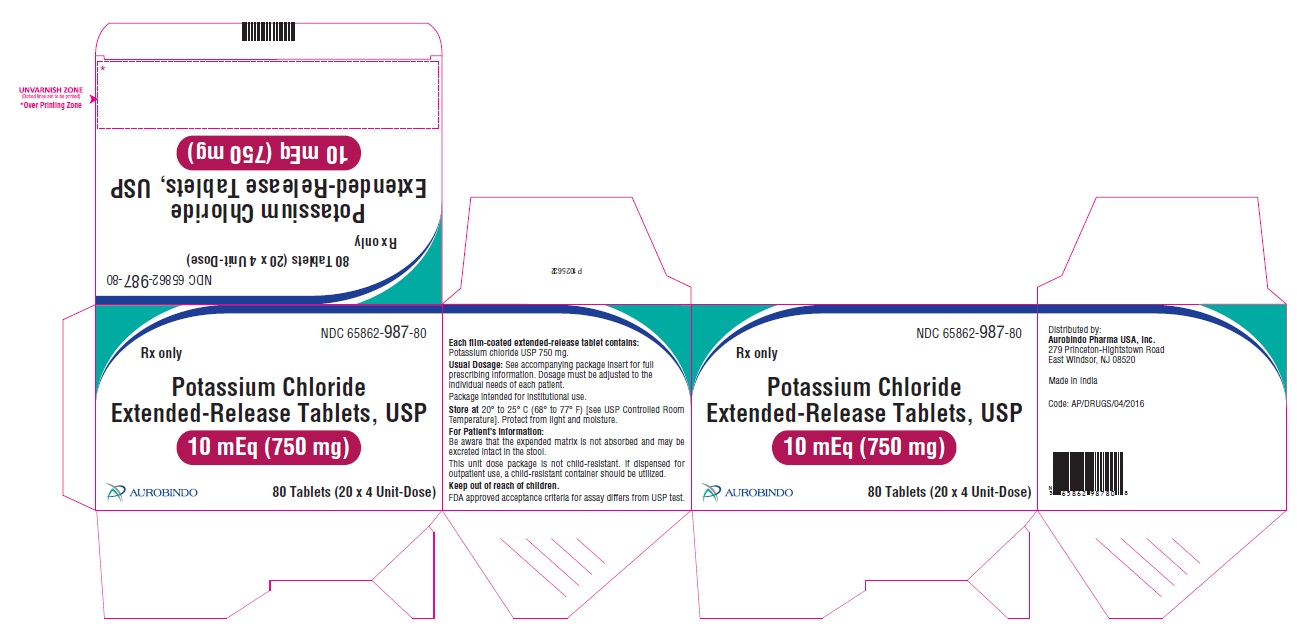
11 DESCRIPTION Potassium chloride extended-release tablets USP are a solid oral dosage form of potassium chloride. Each contains 600 mg or 750 mg of potassium chloride equivalent to 8 mEq or 10 mEq of potassium in a wax matrix tablet. Potassium chloride extended-release tablets USP are an electrolyte replenisher. The chemical name is potassium chloride, and the structural formula is KCl. Potassium chloride, USP is a white, crystalline powder or colorless crystals. It is odorless and has a saline taste. Its solutions are neutral to litmus. It is freely soluble in water and practically insoluble in ethanol. Inactive Ingredients: Colloidal silicon dioxide, hydrogenated vegetable oil, magnesium stearate, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide. In addition, the 8 mEq tablets contain iron oxide red. FDA approved acceptance criteria for assay differs from USP test. Meets USP Dissolution Test 5.
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Potassium Chloride Extended-Release Tablets USP contains 600 mg or 750 mg of potassium chloride equivalent to 8 mEq and 10 mEq respectively. Potassium chloride is provided as extended-release tablets. Potassium Chloride Extended-Release Tablets USP 600 mg (8 mEq) are pink, circular, film-coated biconvex tablet debossed with “N 31” on one side and plain on other side. Bottles of 100 NDC 65862-986-01 Bottles of 500 NDC 65862-986-05 Cartons of 80 Tablets (20 X 4 Unit-Dose) NDC 65862-986-80 Cartons of 120 Tablets (20 X 6 Unit-Dose) NDC 65862-986-12 Potassium Chloride Extended-Release Tablets USP 750 mg (10 mEq) are white to off-white, circular, film-coated biconvex tablet debossed with “N 32” on one side and plain on other side. Bottles of 100 NDC 65862-987-01 Bottles of 1,000 NDC 65862-987-99 Cartons of 80 Tablets (20 X 4 Unit-Dose) NDC 65862-987-80 Cartons of 120 Tablets (20 X 6 Unit-Dose) NDC 65862-987-12 Store at 20 o to 25 o C (68 o to 77 o F) [see USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container with a child-resistant closure.
Indications & Usage
1 INDICATIONS AND USAGE Potassium chloride extended-release tablets are indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis, in patients for whom dietary management with potassium-rich foods or diuretic dose reduction is insufficient. Potassium chloride extended-release tablets are a potassium salt, indicated for the treatment and prophylaxis of hypokalemia with or without metabolic alkalosis in patients for whom dietary management with potassium-rich foods or diuretic dose reduction is insufficient. (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Monitor serum potassium and adjust dosages accordingly (2.1) If serum potassium is less than 2.5 mEq/L, use intravenous potassium instead of oral supplementation (2.1) Take with meals and with a glass of water or other liquid. Swallow tablets whole without crushing, chewing or sucking. (2.1) Treatment of hypokalemia : Doses range from 40 to 100 mEq/day in divided doses. Limit doses to 40 mEq per dose. (2.2) Prevention of hypokalemia : Typical dose is 20 mEq per day. (2.2) 2.1 Administration and Monitoring If serum potassium concentration is less than 2.5 mEq/L, use intravenous potassium instead of oral supplementation. Monitoring Monitor serum potassium and adjust dosages accordingly. Monitor serum potassium periodically during maintenance therapy to ensure potassium remains in desired range. The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease, or acidosis, requires careful attention to acid-base balance, volume status, electrolytes, including magnesium, sodium, chloride, phosphate, and calcium, electrocardiograms, and the clinical status of the patient. Correct volume status, acid-base balance, and electrolyte deficits as appropriate. Administration Take potassium chloride extended-release tablets with meals and with a glass of water or other liquid. Do not take potassium chloride extended-release tablets on an empty stomach because of its potential for gastric irritation [see Warnings and Precautions (5.1) ] . Swallow tablets whole without crushing, chewing or sucking. 2.2 Dosing Dosage must be adjusted to the individual needs of each patient. Dosages greater than 40 mEq per day should be divided such that no more than 40 mEq is given in a single dose. Treatment of Hypokalemia: Typical dose range is 40 to 100 mEq per day. Maintenance or Prophylaxis: Typical dose range is 20 mEq per day.
