Drug Catalog - Product Detail
PRAVASTATIN SODIUM TB 20MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0486-09 | LUPIN PHARMACEUTICALS | 90 | 20MG | TABLET |
PACKAGE FILES
Generic Name
PRAVASTATIN SODIUM
Substance Name
PRAVASTATIN SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077917
Description
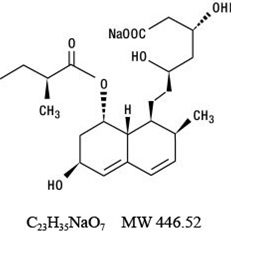
11 DESCRIPTION Pravastatin sodium USP is a statin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Pravastatin sodium is designated chemically as 1-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-b,d,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-, monosodium salt, [1S-[1a(bS*,dS*),2a,6a,8b (R*),8aα]]-. Structural formula: Pravastatin sodium is an odorless, white to off-white, fine or crystalline powder. It is a relatively polar hydrophilic compound with a partition coefficient (octanol/water) of 0.59 at a pH of 7.0. It is soluble in methanol and water (>300 mg/mL), slightly soluble in isopropanol, and practically insoluble in acetone, acetonitrile, chloroform, and ether. Pravastatin sodium tablets USP for oral use contain 10 mg, 20 mg, 40 mg and 80 mg pravastatin sodium, which is equivalent to 9.48, 18.97, 37.94 and 75.88 mg of pravastatin, respectively. Inactive ingredients include: colloidal silicon dioxide, crospovidone, hypromellose, iron oxide yellow, lactose monohydrate, magnesium stearate, polyethylene glycol, sodium bicarbonate, talc and titanium dioxide. image-01
How Supplied
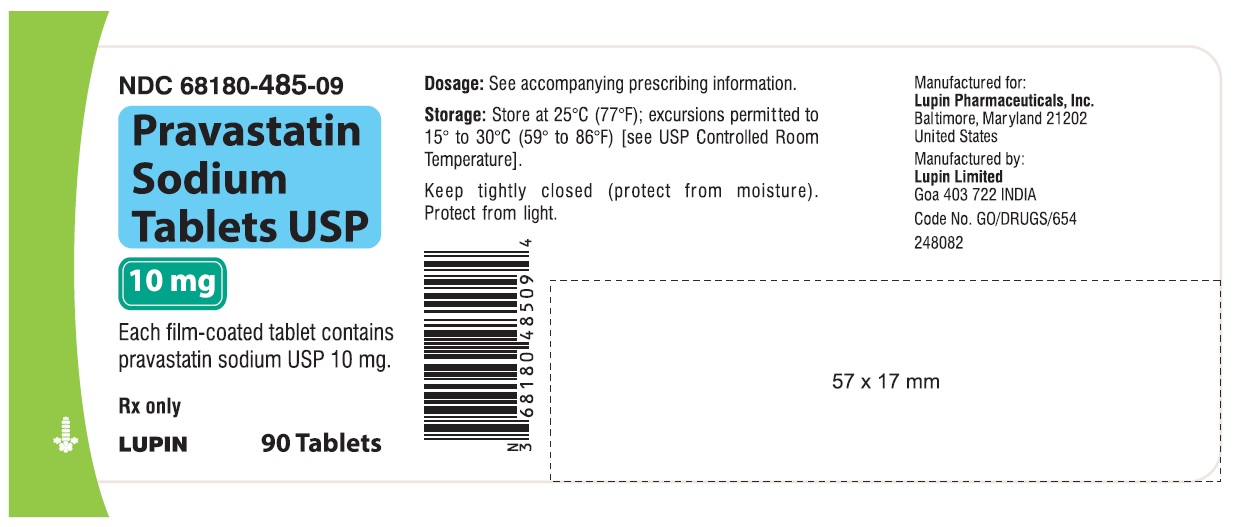
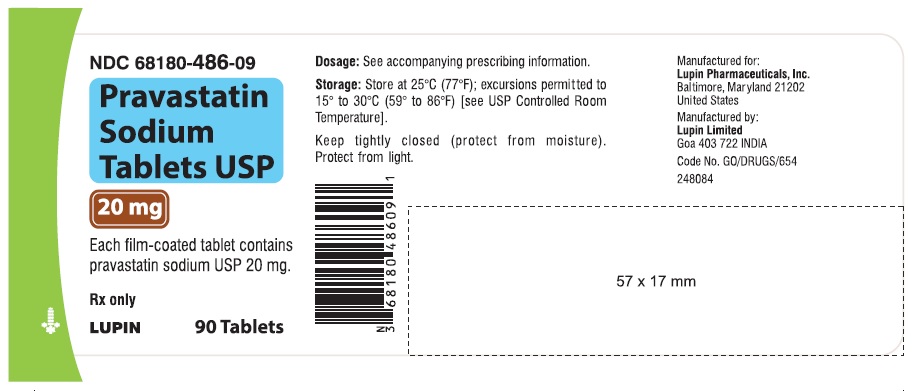
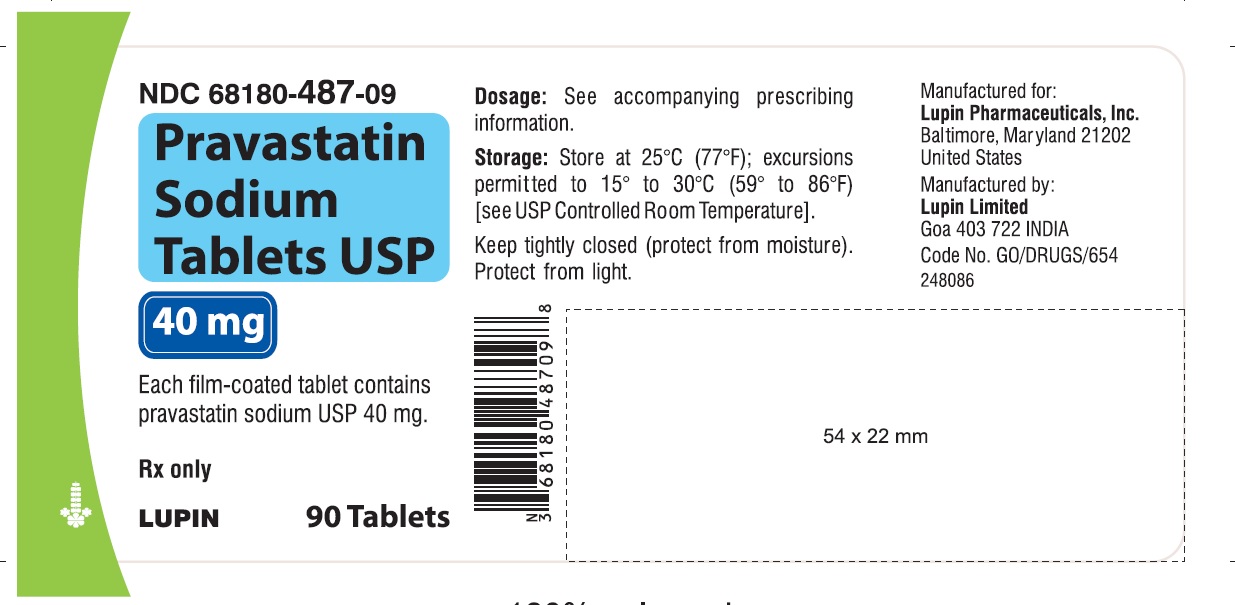
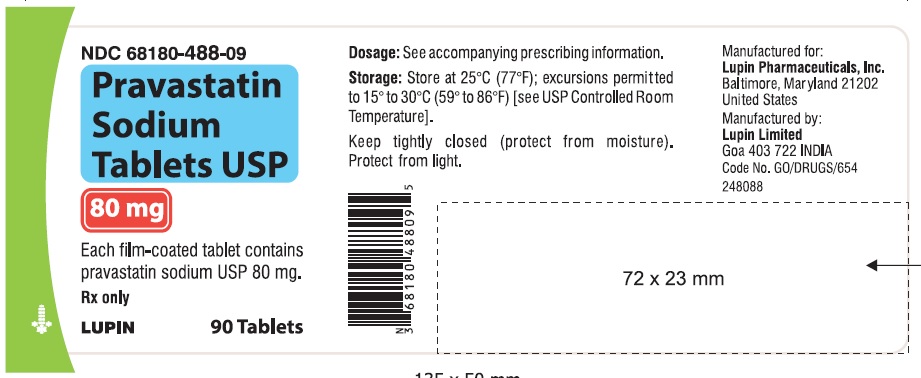
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Pravastatin sodium tablets USP are supplied as: 10 mg tablets: Yellow coloured, capsule shaped, biconvex, film-coated tablets, debossed with 'LU' on one side and 'N01' on the other side. They are supplied in bottles of 90 (NDC 68180-485-09), bottles of 500 (NDC 68180-485-02) and bottles of 1000 (NDC 68180-485-03). 20 mg tablets: Yellow coloured, capsule shaped, biconvex, film-coated tablets, debossed with 'LU' on one side and 'N02' on the other side. They are supplied in bottles of 90 (NDC 68180-486-09), bottles of 500 (NDC 68180-486-02) and bottles of 1000 (NDC 68180-486-03). 40 mg tablets: Yellow coloured, capsule shaped, biconvex, film-coated tablets, debossed with 'LU' on one side and 'N03' on the other side. They are supplied in bottles of 90 (NDC 68180-487-09), bottles of 500 (NDC 68180-487-02) and bottles of 1000 (NDC 68180-487-03). 80 mg tablets: Yellow coloured, oval shaped, biconvex, film-coated tablets, debossed with 'LU' on one side and 'N04' on the other side. They are supplied in bottles of 90 (NDC 68180-488-09), bottles of 500 (NDC 68180-488-02) and bottles of 1000 (NDC 68180-488-03). Store at 25 ο C (77 ο F); excursions permitted to 15 ο C to 30 ο C (59 ο to 86 ο F). Keep tightly closed (protect from moisture).Protect from light.
Indications & Usage
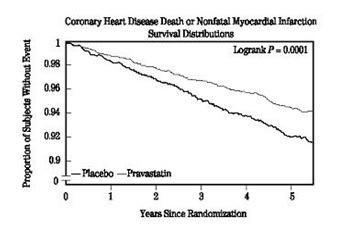
1 INDICATIONS AND USAGE Pravastatin sodium tablets are an HMG-CoA reductase inhibitor (statin) indicated ( 1 ): To reduce the risk of myocardial infarction, myocardial revascularization procedures, and cardiovascular mortality in adults with elevated lowdensity lipoprotein cholesterol (LDL-C) without clinically evident coronary heart disease (CHD). To reduce the risk of coronary death, myocardial infarction, myocardial revascularization procedures, stroke or transient ischemic attack, and slow the progression of coronary atherosclerosis in adults with clinically evident CHD. As an adjunct to diet to reduce LDL-C in adults with primary hyperlipidemia. As an adjunct to diet to reduce LDL-C in pediatric patients ages 8 years and older with heterozygous familial hypercholesterolemia (HeFH). As an adjunct to diet for the treatment of adults with: Primary dysbetalipoproteinemia. Hypertriglyceridemia. Pravastatin sodium tablets are indicated: To reduce the risk of myocardial infarction, myocardial revascularization procedures, and cardiovascular mortality in adults with elevated low-density lipoprotein cholesterol (LDLC) without clinically evident coronary heart disease (CHD). To reduce the risk of coronary death, myocardial infarction, myocardial revascularization procedures, stroke or transient ischemic attack, and slow the progression of coronary atherosclerosis in adults with clinically evident CHD. As an adjunct to diet to reduce LDL-C in adults with primary hyperlipidemia. As an adjunct to diet to reduce LDL-C in pediatric patients ages 8 years and older with heterozygous familial hypercholesterolemia (HeFH). As an adjunct to diet for the treatment of adults with: Primary dysbetalipoproteinemia. Hypertriglyceridemia.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Take orally once daily at any time of the day, with or without food ( 2.1 ). For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving pravastatin sodium 80 mg daily, prescribe alternative LDL-C-lowering treatment ( 2.1 ). Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating pravastatin sodium and adjust the dosage if necessary ( 2.1 ). Adults: recommended starting dosage is pravastatin sodium 40 mg to 80 mg once daily ( 2.2 ). Pediatric Patients ( 2.3 ): aged 8 to 13 years, the recommended dosage is 20 mg once daily. aged 14 to 18 years, the recommended starting dosage is 40 mg once daily. Severe renal impairment: recommended starting dosage is pravastatin sodium 10 mg once daily. Recommended maximum pravastatin sodium dosage is 40 mg once daily ( 2.4 ). See full prescribing information for dosage modifications due to drug interactions ( 2.5 , 7 ). 2.1 Important Dosage and Administration Information Take pravastatin sodium tablets orally once daily as a single dose at any time of the day, with or without food. For patients that require a high-intensity statin or are unable to achieve their LDL-C goal receiving pravastatin sodium tablets 80 mg daily, prescribe alternative LDL-C-lowering treatment. Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating pravastatin sodium tablets, and adjust the dosage if necessary. 2.2 Recommended Dosage in Adult Patients The recommended starting dose is 40 mg to 80 mg once daily. 2.3 Recommended Dosage in Pediatric Patients 8 Years of Age and Older with HeFH In pediatric patients aged 8 to 13 years, the recommended dosage is pravastatin sodium 20 mg once daily. In pediatric patients aged 14 to 18 years, the recommended starting dosage is pravastatin sodium 40 mg once daily. 2.4 Recommended Dosage in Patients with Renal Impairment In patients with severe renal impairment, the recommended starting dosage is pravastatin sodium 10 mg once daily. The maximum recommended dosage of pravastatin sodium in patients with severe renal impairment is 40 mg once daily [see Clinical Pharmacology ( 12.3 )]. The recommended dosage of pravastatin sodium for patients with mild or moderate renal impairment is the same as patients with normal renal function. 2.5 Dosage and Administration Modifications Due to Drug Interactions In patients taking a bile acid sequestrant, administer pravastatin sodium at least 1 hour before or 4 hours after the bile acid sequestrant [See Drug Interactions ( 7.2 )]. Concomitant use of pravastatin sodium with the following drugs requires dosage modifications of pravastatin sodium [see Warnings and Precautions ( 5.1 ) and Drug Interactions ( 7.1 )] : о Cyclosporine In patients taking cyclosporine, the recommended starting dosage is pravastatin sodium 10 mg once daily. The maximum recommended dosage of pravastatin sodium in patients taking cyclosporine is 20 mg once daily. о Clarithromycin and Erythromycin The maximum recommended dosage is pravastatin sodium 40 mg once daily.
