Drug Catalog - Product Detail
PREDNISOLONE ACETATE OPHTH SUSP 1% 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 60758-0119-05 | GREENSTONE | 5 | 1% | SUSPENSION |
PACKAGE FILES


Generic Name
PREDNISOLONE ACETATE
Substance Name
PREDNISOLONE ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
NDA017011
Description
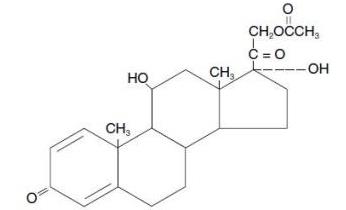
DESCRIPTION Prednisolone acetate ophthalmic suspension, USP 1% is a sterile, topical anti-inflammatory agent for ophthalmic use. Its chemical name is 11ß,17, 21-Trihydroxypregna-1,4-diene-3, 20-dione 21-acetate and it has the following structure: Structural Formula: prednisolone acetate Each mL of Prednisolone acetate ophthalmic suspension 1% contains: Active : prednisolone acetate (microfine suspension) 1% Inactives : benzalkonium chloride as preservative; boric acid; edetate disodium; hypromellose; polysorbate 80; purified water; sodium bisulfite; sodium chloride; and sodium citrate. The pH during its shelf life ranges from 5.0 - 6.0. The following structure for Prednisolone acetate ophthalmic suspension, USP 1% is a sterile, topical anti-inflammatory agent for ophthalmic use. Its chemical name is 11ß,17, 21-Trihydroxypregna-1,4-diene-3, 20-dione 21-acetate.
How Supplied
HOW SUPPLIED Prednisolone acetate ophthalmic suspension, USP 1% is supplied sterile in opaque white LDPE plastic bottles with droppers with pink high impact polystyrene (HIPS) caps as follows: 5 mL in 10 mL bottle - NDC 60758-119-05 10 mL in 15 mL bottle - NDC 60758-119-10 15 mL in 15 mL bottle - NDC 60758-119-15 Pacific Pharma Storage : Store at up to 25°C (77°F). Protect from freezing. Store in an upright position. Revised: 0 3 /202 4 Distributed by: AbbVie Inc. North Chicago, IL 60064 © 2024 AbbVie. All rights reserved. PACIFIC PHARMA and its design are trademarks of Allergan, Inc., an AbbVie company. v2.0USPI0119
Indications & Usage
INDICATIONS AND USAGE Prednisolone acetate ophthalmic suspension 1% is indicated for the treatment of steroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe.
Dosage and Administration
DOSAGE AND ADMINISTRATION Shake well before using. Instill one to two drops into the conjunctival sac two to four times daily. During the initial 24 to 48 hours, the dosing frequency may be increased if necessary. Care should be taken not to discontinue therapy prematurely. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated (see PRECAUTIONS ).
