Drug Catalog - Product Detail
PREZCOBIX 150-800MG TAB 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 59676-0575-30 | JANSSEN PRODUCTS | 30 | 800-150MG | TABLET |
PACKAGE FILES


Generic Name
DARUNAVIR ETHANOLATE AND COBICISTAT
Substance Name
COBICISTAT
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA205395
Description
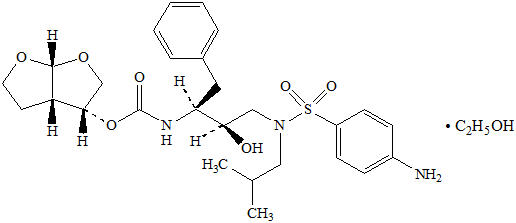
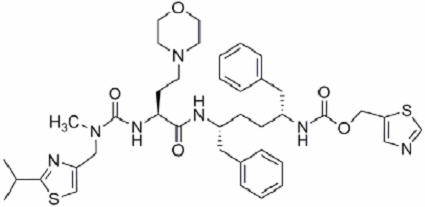
11 DESCRIPTION PREZCOBIX ® is a fixed-dose combination tablet containing darunavir and cobicistat. Darunavir is an inhibitor of the human immunodeficiency virus (HIV-1) protease. Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family. PREZCOBIX ® 800 mg darunavir/150 mg cobicistat tablets are for oral administration. Each tablet contains darunavir ethanolate equivalent to 800 mg of darunavir and 150 mg of cobicistat. The tablets include the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, and silicified microcrystalline cellulose. The tablets are film-coated with a coating material containing iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide. PREZCOBIX ® 675 mg darunavir/150 mg cobicistat tablets are for oral administration. Each tablet contains darunavir ethanolate equivalent to 675 mg of darunavir and 150 mg of cobicistat. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing iron oxide black, iron oxide yellow, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide. Darunavir : Darunavir, in the form of darunavir ethanolate, has the following chemical name: [(1 S ,2 R )-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3 R ,3a S ,6a R )-hexahydrofuro[2,3- b ]furan-3-yl ester monoethanolate. Its molecular formula is C 27 H 37 N 3 O 7 S ∙ C 2 H 5 OH and its molecular weight is 593.73. Darunavir ethanolate has the following structural formula: Chemical Structure Cobicistat : Cobicistat is adsorbed onto silicon dioxide. The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl[(2 R , 5 R )-5-{[(2 S )2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4yl)butanoyl]amino}-1,6-diphenylhexan-2-yl] carbamate. It has a molecular formula of C 40 H 53 N 7 O 5 S 2 and a molecular weight of 776.0. It has the following structural formula: Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING PREZCOBIX ® (darunavir and cobicistat) tablets, 800/150 mg, are supplied as pink, oval-shaped, film-coated tablets debossed with "800" on one side and "TG" on the other side. Bottle of 30 tablets (NDC 59676-575-30). PREZCOBIX ® (darunavir and cobicistat) tablets, 675/150 mg, are supplied as green to dark green, oval-shaped, scored film-coated tablet debossed with “675” on one side and “TG” on the other side. Bottle of 30 tablets (NDC 59676-578-30). Storage: Store at 20 °C–25 °C (between 68 °F–77 °F); with excursions permitted to 15 °C–30 °C (59 °F–86 °F) [see USP Controlled Room Temperature]. Keep PREZCOBIX and all medicines out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE PREZCOBIX is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) in treatment-naïve and treatment-experienced adults and pediatric patients weighing at least 25 kg with no darunavir resistance-associated substitutions (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, L89V). PREZCOBIX is a two-drug combination of darunavir, a human immunodeficiency virus (HIV-1) protease inhibitor, and cobicistat, a CYP3A inhibitor, and is indicated for the treatment of HIV-1 in treatment-naïve and treatment-experienced adults and pediatric patients weighing at least 25 kg with no darunavir resistance-associated substitutions (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, L89V). ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Recommended dosage: Adults and pediatric patients weighing at least 40 kg: One 800 mg/150 mg tablet taken once daily with food. ( 2.1 ) Pediatric patients weighing at least 25 kg to less than 40 kg: One 675 mg/150 mg tablet taken once daily with food. ( 2.1 ) Testing Prior to Initiation: HIV genotypic testing is recommended for antiretroviral treatment experienced patients. Assess estimated creatinine clearance in all patients prior to starting PREZCOBIX. When used with tenofovir DF: Assess urine glucose and urine protein at baseline and monitor creatinine clearance, urine glucose, and urine protein. Monitor serum phosphorus in patients with or at risk for renal impairment. ( 2.2 ) 2.1 Recommended Dosage in Adults and Pediatric Patients Weighing at Least 25 kg The recommended dosages of PREZCOBIX for adults and pediatric patients weighing at least 25 kg are shown in Table 1. The pediatric dose is based on weight. Administer PREZCOBIX orally with food in conjunction with other antiretroviral agents. Table 1: Recommended Dosages of PREZCOBIX in Adults and Pediatric Patients Weighing at Least 25 kg, who are Treatment-Naïve or Treatment-Experienced Without DRV RAMs DRV-resistance-associated mutations (RAMs): V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V, L89V. Patient Population Dose (once daily with food) Adult Patients One 800 mg darunavir/150 mg cobicistat tablet Pediatric Patients weighing at least 40 kg Pediatric Patients weighing at least 25 kg to less than 40 kg One 675 mg darunavir/150 mg cobicistat tablet Before prescribing PREZCOBIX 675 mg/150 mg tablets, children should be assessed for the ability to swallow tablets. For patients unable to swallow the 675 mg/150 mg tablet whole, the scored tablet may be split by hand into two pieces. Each piece should be consumed immediately after splitting to ensure the entire dose is administered. The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. 2.2 Testing Prior to Initiation of PREZCOBIX HIV Genotypic Testing HIV genotypic testing is recommended for antiretroviral treatment-experienced patients. However, when HIV genotypic testing is not feasible, PREZCOBIX can be used in protease inhibitor-naïve patients, but is not recommended in protease inhibitor-experienced patients. Creatinine Clearance Prior to starting PREZCOBIX, assess estimated creatinine clearance because cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function [see Warnings and Precautions (5.3) ] . When co-administering PREZCOBIX with tenofovir disoproxil fumarate (tenofovir DF) assess estimated creatinine clearance, urine glucose, and urine protein at baseline [see Warnings and Precautions (5.4) ] . 2.3 Not Recommended in Severe Renal Impairment PREZCOBIX co-administered with tenofovir DF is not recommended in patients who have an estimated creatinine clearance below 70 mL per minute [see Warnings and Precautions (5.4) and Adverse Reactions (6.1) ] . 2.4 Not Recommended in Severe Hepatic Impairment PREZCOBIX is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] . 2.5 Not Recommended During Pregnancy PREZCOBIX is not recommended during pregnancy because of substantially lower exposures of darunavir and cobicistat during the second and third trimesters [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3) ] . PREZCOBIX should not be initiated in pregnant individuals. An alternative regimen is recommended for those who become pregnant during therapy with PREZCOBIX.
