Drug Catalog - Product Detail
PRIMIDONE Tab 250 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0545-10 | AMNEAL PHARMACEUTICALS | 100 | 250MG | TABLET |
PACKAGE FILES

Generic Name
PRIMIDONE
Substance Name
PRIMIDONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040866
Description
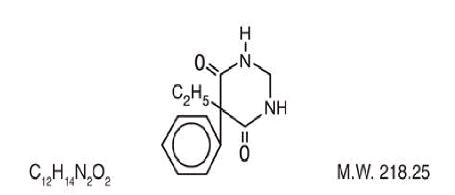
DESCRIPTION Primidone, USP is a white, crystalline, highly stable substance, M.P. 279-284° C. It is poorly soluble in water (60 mg per 100 mL at 37° C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog. Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula: Primidone tablets USP, 50 mg and 250 mg contain the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate, methyl cellulose, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate. 36e4a4a6-figure-01
How Supplied
HOW SUPPLIED Primidone Tablets, USP, 50 mg are supplied as white, round, flat-faced, bevel-edged tablets, debossed “AN” above “44” on one side and cut-bisected on the other side. They are available as follows: Bottles of 100: NDC 65162-544-10 Bottles of 500: NDC 65162-544-50 Bottles of 1000: NDC 65162-544-11 Primidone Tablets, USP, 250 mg are supplied as white, round, flat-faced, bevel-edged tablets, debossed “AN” bisect “545” on one side and plain on the other side. They are available as follows: Bottles of 100: NDC 65162-545-10 Bottles of 500 : NDC 65162-545-50 Bottles of 1000: NDC 65162-545-11 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Dispense contents with a child-resistant closure (as required) and in a tight, light-resistant container as defined in the USP. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Rev. 12-2021-03
Indications & Usage
INDICATIONS AND USAGE Primidone tablets used alone or concomitantly with other anticonvulsants, are indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Usual Dosage Patients 8 years of age and older who have received no previous treatment may be started on primidone tablets according to the following regimen using either 50 mg or scored 250 mg primidone tablets: Days 1 to 3: 100 to 125 mg at bedtime. Days 4 to 6: 100 to 125 mg twice a day. Days 7 to 9: 100 to 125 mg three times a day. Day 10 to maintenance: 250 mg three times a day. For most adults and children 8 years of age and over, the usual maintenance dosage is three to four 250 mg primidone tablets in divided doses (250 mg three times a day or four times a day). If required, an increase to five or six 250 mg tablets daily may be made, but daily doses should not exceed 500 mg four times a day. Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations of primidone may be necessary for optimal dosage adjustment. The clinically effective serum level for primidone is between 5 to 12 mcg/mL. INITIAL: ADULTS AND CHILDREN OVER 8 KEY: •=50 mg tablet; ●=250 mg tablet DAY 1 2 3 4 5 6 AM •• •• •• NOON PM •• •• •• •• •• •• DAY 7 8 9 10 11 12 AM •• •• •• ● Adjust to Maintenance NOON •• •• •• ● PM •• •• •• ● Patients Already Receiving Other Anticonvulsants Primidone tablets should be started at 100 to 125 mg at bedtime and gradually increased to maintenance level as the other drug is gradually decreased. This regimen should be continued until satisfactory dosage level is achieved for the combination, or the other medication is completely withdrawn. When therapy with primidone tablets alone is the objective, the transition from concomitant therapy should not be completed in less than two weeks. Pediatric Dosage For children under 8 years of age, the following regimen may be used: Days 1 to 3: 50 mg at bedtime. Days 4 to 6: 50 mg twice a day. Days 7 to 9: 100 mg twice a day. Day 10 to maintenance: 125 mg three times a day to 250 mg three times a day. For children under 8 years of age, the usual maintenance dosage is 125 to 250 mg three times daily or, 10 to 25 mg/kg/day in divided doses.
