Drug Catalog - Product Detail
PRIMIDONE TAB 250MG 1000CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00527-1231-10 | LANNETT | 1000 | 250MG | TABLET |
PACKAGE FILES

Generic Name
PRIMIDONE
Substance Name
PRIMIDONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA084903
Description
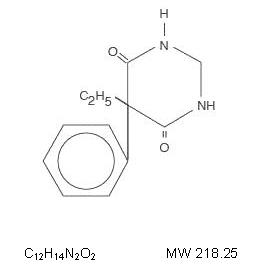
DESCRIPTION Anticonvulsant Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1 H ,5 H )-pyrimidinedione. Structural formula: Primidone is a white, crystalline, highly stable substance, M.P. 279 - 284°C. It is poorly soluble in water (60 mg per 100 mL at 37°C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog. Each tablet, for oral administration, contains either 50 mg or 250 mg of primidone. In addition, each tablet contains the following inactive ingredients: lactose monohydrate, methylcellulose, acacia, sodium starch glycolate, and magnesium stearate. primidone-molecular-structure
How Supplied
HOW SUPPLIED Primidone Tablets USP, 50 mg are available as white, round, flat faced, beveled edge, scored tablets debossed LAN over 1301, supplied in bottles of 100, 500 and 1000 tablets. 100 Tablets NDC 0527-1301-01 500 Tablets NDC 0527-1301-05 1000 Tablets NDC 0527-1301-10 Primidone Tablets USP, 250 mg are available as white, round, flat faced, beveled edge, scored tablets debossed LAN over 1231, supplied in bottles of 100, 500 and 1000 tablets. 100 Tablets NDC 0527-1231-01 500 Tablets NDC 0527-1231-05 1000 Tablets NDC 0527-1231-10 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP with a child-resistant closure. Distributed by: Lannett Company, Inc. Philadelphia, PA 19136 Dispense with Medication Guide available at: www.lannett.com/med-guide/primidone CIB70303E Rev. 06/20
Indications & Usage
INDICATIONS AND USAGE Primidone, used alone or concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adult Dosage Patients 8 years of age and older who have received no previous treatment may be started on primidone according to the following regimen using either 50 mg or scored 250 mg primidone tablets: Days 1 to 3: 100 to 125 mg at bedtime. Days 4 to 6: 100 to 125 mg b.i.d. Days 7 to 9: 100 to 125 mg t.i.d. Day 10 to maintenance: 250 mg t.i.d. For most adults and children 8 years of age and over, the usual maintenance dosage is three to four 250 mg primidone tablets in divided doses (250 mg t.i.d. or q.i.d.). If required, an increase to five or six 250 mg tablets daily may be made but daily doses should not exceed 500 mg q.i.d. INITIAL: ADULTS AND CHILDREN OVER 8 KEY: * = 50 mg tablet; • = 250 mg tablet DAY 1 2 3 4 5 6 AM ** ** ** NOON PM ** ** ** ** ** ** DAY 7 8 9 10 11 12 AM ** ** ** • Adjust to Maintenance NOON ** ** ** • PM ** ** ** • Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations of primidone may be necessary for optimal dosage adjustment. The clinically effective serum level for primidone is between 5 to 12 μg/mL. In Patients Already Receiving Other Anticonvulsants Primidone should be started at 100 to 125 mg at bedtime and gradually increased to maintenance level as the other drug is gradually decreased. This regimen should be continued until satisfactory dosage level is achieved for the combination, or the other medication is completely withdrawn. When therapy with primidone alone is the objective, the transition from concomitant therapy should not be completed in less than two weeks. Pediatric Dosage For children under 8 years of age, the following regimen may be used: Days 1 to 3: 50 mg at bedtime. Days 4 to 6: 50 mg b.i.d. Days 7 to 9: 100 mg b.i.d. Day 10 to maintenance: 125 mg t.i.d. to 250 mg t.i.d. For children under 8 years of age, the usual maintenance dosage is 125 to 250 mg three times daily or, 10 to 25 mg/kg/day in divided doses.
