Drug Catalog - Product Detail
QUINAPRIL HCL 40MG TB 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0620-90 | AUROBINDO PHARMA | 90 | 40MG | TABLET |
PACKAGE FILES

Generic Name
QUINAPRIL HYDROCHLORIDE
Substance Name
QUINAPRIL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202725
Description
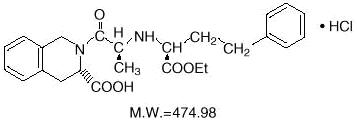
DESCRIPTION Quinapril hydrochloride is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat. Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, monohydrochloride. Its molecular formula is C 25 H 30 N 2 O 5 •HCl and its structural formula is: Quinapril hydrochloride USP is a white to off-white powder, with a pink cast at times that is freely soluble in aqueous solvents. Quinapril tablets, USP contain 5 mg (equivalent to 5.416 mg quinapril hydrochloride), 10 mg (equivalent to 10.832 mg quinapril hydrochloride), 20 mg (equivalent to 21.664 mg quinapril hydrochloride), or 40 mg (equivalent to 43.328 mg quinapril hydrochloride) of quinapril for oral administration. Each tablet also contains colloidal silicon dioxide, crospovidone, hydroxypropyl cellulose, hypromellose, iron oxide red, lactose monohydrate, magnesium carbonate, magnesium stearate, polyethylene glycol, povidone, and titanium dioxide. Chemical Structure
How Supplied
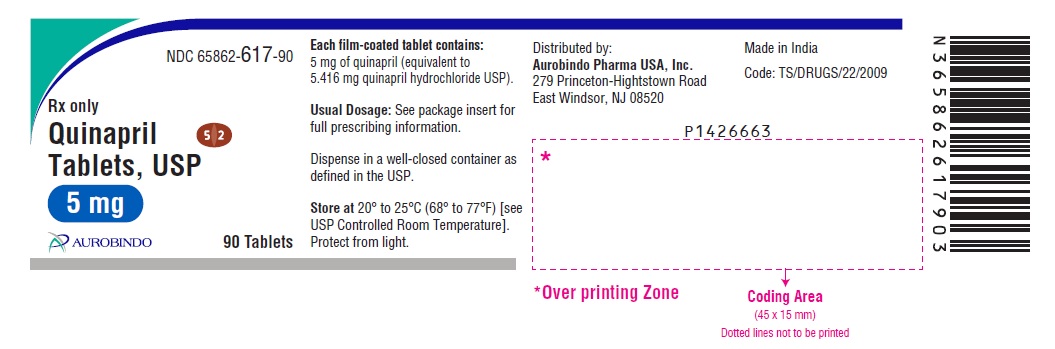
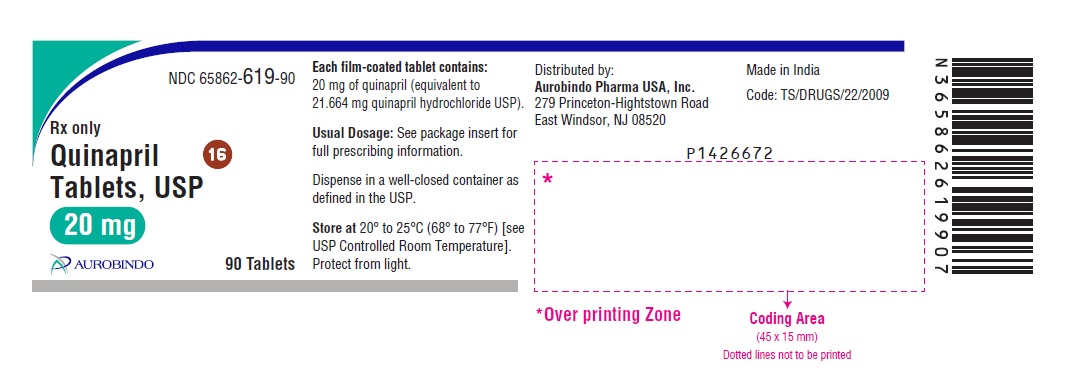
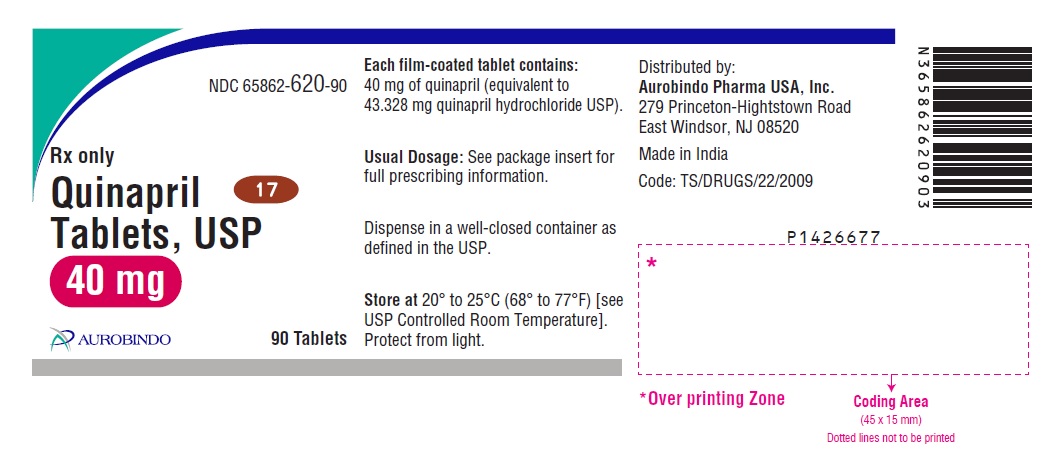
HOW SUPPLIED Quinapril tablets, USP are supplied as follows: Quinapril Tablets USP, 5 mg are brown colored, oval shaped film-coated tablets, debossed with “5” and “2” on either side of the scoreline on one side and “H” on the other side. Bottles of 30 NDC 65862-617-30 Bottles of 90 NDC 65862-617-90 Bottles of 1,000 NDC 65862-617-99 Bottles of 15,000 NDC 65862-617-55 Cartons of 100 (10 x 10) Unit-dose Tablets NDC 65862-617-78 Quinapril Tablets USP, 10 mg are brown colored, triangular shaped film-coated tablets, debossed with “53” on one side and “H” on the other side. Bottles of 30 NDC 65862-618-30 Bottles of 90 NDC 65862-618-90 Bottles of 1,000 NDC 65862-618-99 Bottles of 15,000 NDC 65862-618-55 Cartons of 100 (10 x 10) Unit-dose Tablets NDC 65862-618-78 Quinapril Tablets USP, 20 mg are brown colored, circular shaped film-coated tablets, debossed with “D” on one side and “16” on the other side. Bottles of 30 NDC 65862-619-30 Bottles of 90 NDC 65862-619-90 Bottles of 1,000 NDC 65862-619-99 Bottles of 10,000 NDC 65862-619-19 Cartons of 100 (10 x 10) Unit-dose Tablets NDC 65862-619-78 Quinapril Tablets USP, 40 mg are brown colored, oval shaped film-coated tablets, debossed with “D” on one side and “17” on the other side. Bottles of 30 NDC 65862-620-30 Bottles of 90 NDC 65862-620-90 Bottles of 1,000 NDC 65862-620-99 Bottles of 3,000 NDC 65862-620-39 Cartons of 100 (10 x 10) Unit-dose Tablets NDC 65862-620-78 Dispense in well-closed containers as defined in the USP. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Distributed by: Aurobindo Pharma USA, Inc. 279 Princeton-Hightstown Road East Windsor, NJ 08520 Manufactured by: Aurobindo Pharma Limited Hyderabad-500 038, India Revised: 07/2020
Indications & Usage
INDICATIONS AND USAGE Hypertension Quinapril tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with quinapril tablets, USP. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Quinapril tablets, USP may be used alone or in combination with thiazide diuretics . Heart Failure Quinapril tablets, USP are indicated in the management of heart failure as adjunctive therapy when added to conventional therapy including diuretics and/or digitalis. In using quinapril tablets, USP consideration should be given to the fact that another ACE inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease. Available data are insufficient to show that quinapril tablets, USP do not have a similar risk (see WARNINGS ). Angioedema in black patients: Black patients receiving ACE inhibitor monotherapy have been reported to have a higher incidence of angioedema compared to non-blacks. It should also be noted that in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks.
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension Monotherapy The recommended initial dosage of quinapril tablets in patients not on diuretics is 10 or 20 mg once daily. Dosage should be adjusted according to blood pressure response measured at peak (2 to 6 hours after dosing) and trough (predosing). Generally, dosage adjustments should be made at intervals of at least 2 weeks. Most patients have required dosages of 20, 40, or 80 mg/day, given as a single dose or in two equally divided doses. In some patients treated once daily, the antihypertensive effect may diminish toward the end of the dosing interval. In such patients an increase in dosage or twice daily administration may be warranted. In general, doses of 40 to 80 mg and divided doses give a somewhat greater effect at the end of the dosing interval. Concomitant Diuretics If blood pressure is not adequately controlled with quinapril tablets monotherapy, a diuretic may be added. In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of quinapril tablets. To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued 2 to 3 days prior to beginning therapy with quinapril tablets (see WARNINGS ). Then, if blood pressure is not controlled with quinapril tablets alone, diuretic therapy should be resumed. If the diuretic cannot be discontinued, an initial dose of 5 mg quinapril tablets should be used with careful medical supervision for several hours and until blood pressure has stabilized. The dosage should subsequently be titrated (as described above) to the optimal response (see WARNINGS, PRECAUTIONS, and Drug Interactions ). Renal Impairment Kinetic data indicate that the apparent elimination half-life of quinaprilat increases as creatinine clearance decreases. Recommended starting doses, based on clinical and pharmacokinetic data from patients with renal impairment, are as follows: Creatinine Clearance Maximum Recommended Initial Dose >60 mL/min 10 mg 30 to 60 mL/min 5 mg 10 to 30 mL/min 2.5 mg <10 mL/min Insufficient data for dosage recommendation Patients should subsequently have their dosage titrated (as described above) to the optimal response. Elderly (≥65 years) The recommended initial dosage of quinapril tablets in elderly patients is 10 mg given once daily followed by titration (as described above) to the optimal response. Heart Failure Quinapril tablets are indicated as adjunctive therapy when added to conventional therapy including diuretics and/or digitalis. The recommended starting dose is 5 mg twice daily. This dose may improve symptoms of heart failure, but increases in exercise duration have generally required higher doses. Therefore, if the initial dosage of quinapril tablets is well tolerated, patients should then be titrated at weekly intervals until an effective dose, usually 20 to 40 mg daily given in two equally divided doses, is reached or undesirable hypotension, orthostatis, or azotemia (see WARNINGS ) prohibit reaching this dose. Following the initial dose of quinapril tablets, the patient should be observed under medical supervision for at least two hours for the presence of hypotension or orthostatis and, if present, until blood pressure stabilizes. The appearance of hypotension, orthostatis, or azotemia early in dose titration should not preclude further careful dose titration. Consideration should be given to reducing the dose of concomitant diuretics. DOSE ADJUSTMENTS IN PATIENTS WITH HEART FAILURE AND RENAL IMPAIRMENT OR HYPONATREMIA Pharmacokinetic data indicate that quinapril elimination is dependent on level of renal function. In patients with heart failure and renal impairment, the recommended initial dose of quinapril tablets is 5 mg in patients with a creatinine clearance above 30 mL/min and 2.5 mg in patients with a creatinine clearance of 10 to 30 mL/min. There is insufficient data for dosage recommendation in patients with a creatinine clearance less than 10 mL/min (see DOSAGE AND ADMINISTRATION , Heart Failure, WARNINGS, and PRECAUTIONS, Drug Interactions ). If the initial dose is well tolerated, quinapril tablets may be administered the following day as a twice daily regimen. In the absence of excessive hypotension or significant deterioration of renal function, the dose may be increased at weekly intervals based on clinical and hemodynamic response.
