Drug Catalog - Product Detail
QUINIDINE SULFATE TB 200MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00185-4346-01 | SANDOZ | 100 | 200MG | TABLET |
PACKAGE FILES


Generic Name
QUINIDINE SULFATE
Substance Name
QUINIDINE SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA088072
Description
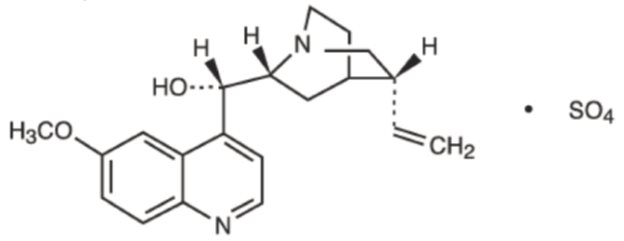
DESCRIPTION Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the d- isomer of quinine, and its molecular weight is 324.43. Quinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,6’- methoxy-,(9 S )-, sulfate(2:1) dihydrate; its structural formula is: Its molecular formula is: C 40 H 48 N 4 O 4 •H 2 SO 4 •2H 2 O; and its molecular weight is 782.96, of which 82.9% is quinidine base. Quinidine sulfate occurs as fine needle-like, white crystals, frequently cohering in masses, or fine, white powder. It is odorless, has a very bitter taste, and darkens on exposure to light. It is slightly soluble in water, soluble in alcohol and in chloroform, and insoluble in ether. Each tablet, for oral administration, contains 200 mg of quinidine sulfate (equivalent to 166 mg of quinidine base) 300 mg of quinidine sulfate (equivalent to 249 mg of quinidine base). In addition, each tablet contains the following inactive ingredients: confectioner’s sugar, corn starch, microcrystalline cellulose, pregelatinized starch and zinc stearate. Chemical Structure
How Supplied
HOW SUPPLIED Quinidine Sulfate Tablets are supplied as follows: 200 mg - White tablet scored imprinted E511 NDC 0185-4346-01 bottles of 100 NDC 0185-4346-10 bottles of 1000 300 mg - White tablet scored imprinted E512 NDC 0185-1047-01 bottles of 100 NDC 0185-1047-10 bottles of 1000 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a well-closed, light-resistant container. KEEP OUT OF THE REACH OF CHILDREN. Manufactured for Sandoz Inc. Princeton, NJ 08540 Manufactured by Epic Pharma, LLC Laurelton, NY 11413 Rev. August 2019 MF1047REV08/19
Indications & Usage
Dosage and Administration
DOSAGE AND ADMINISTRATION Treatment of P. Falcipum Malaria Quinidine sulfate tablets are used in one of the approved regimens for the treatment of life-threatening P. falciparum malaria. The central component of the regimen is Quinidine Gluconate Injection, and the regimen is described in the package insert of Quinidine Gluconate Injection. Conversion of Atrial Fibrillation/Flutter to Sinus Rhythm Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine sulfate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Patients with symptomatic atrial fibrillation/ flutter should be treated with quinidine sulfate only after ventricular rate control ( e.g., with digitalis or β-blockers) has failed to provide satisfactory control of symptoms. Adequate trials have not identified an optimal regimen of quinidine sulfate for conversion of atrial fibrillation/flutter to sinus rhythm. In one reported regimen, the patient first receives two tablets (400 mg; 332 mg of quinidine base) of quinidine sulfate every six hours. If this regimen has not resulted in conversion after 4 or 5 doses, then the dose is cautiously increased. If, at any point during administration, the QRS complex widens to 130% of its pre-treatment duration; the QT C interval widens to 130% of its pre-treatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension, then quinidine sulfate is discontinued, and other means of conversion ( e.g., direct-current cardioversion) are considered. Reduction of Frequency of Relapse Into Atrial Fibrillation/Flutter In a patient with a history of frequent symptomatic episodes of atrial fibrillation/flutter, the goal of therapy with quinidine sulfate should be an increase in the average time between episodes. In most patients, the tachyarrhythmia will recur during therapy with quinidine sulfate, and a single recurrence should not be interpreted as therapeutic failure. Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine sulfate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Monitoring should be continued for two or three days after initiation of the regimen on which the patient will be discharged. Therapy with quinidine sulfate should be begun with 200 mg (equivalent to 166 mg of quinidine base) every six hours. If this regimen is well tolerated, if the serum quinidine level is still well within the laboratory’s therapeutic range, and if the average time between arrhythmic episodes has not been satisfactorily increased, then the dose may be cautiously raised. The total daily dosage should be reduced if the QRS complex widens to 130% of its pretreatment duration; the QT C interval widens to 130% of its pretreatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension. Suppression of Ventricular Arrhythmias Dosing regimens for the use of quinidine sulfate in suppressing life-threatening ventricular arrhythmias have not been adequately studied. Described regimens have generally been similar to the regimen described just above for the prophylaxis of symptomatic atrial fibrillation/flutter. Where possible, therapy should be guided by the results of programmed electrical stimulation and/or Holter monitoring with exercise.
