Drug Catalog - Product Detail
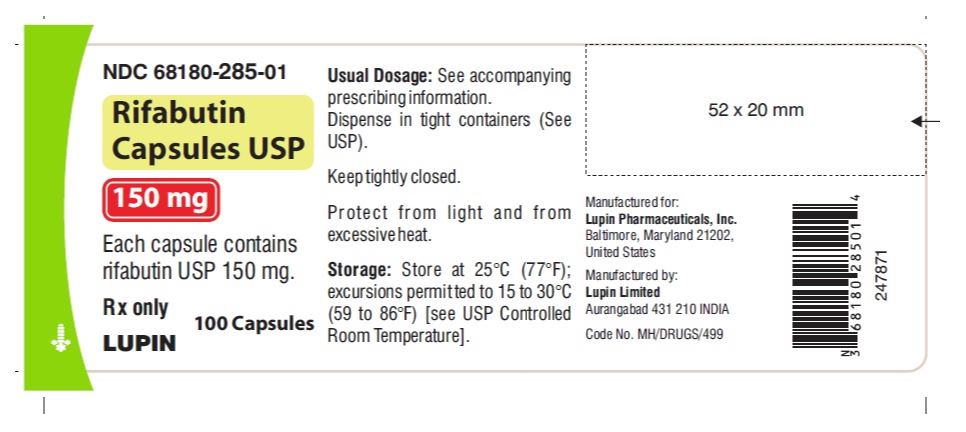
RIFABUTIN CP 150MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0285-01 | LUPIN PHARMACEUTICALS | 100 | 150MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
DESCRIPTION Rifabutin Capsules USP for oral administration contain 150 mg of the rifamycin antimycobacterial agent rifabutin, USP, per capsule, along with the inactive ingredients crospovidone, gelatin, iron oxide red, magnesium stearate, microcrystalline cellulose, potassium hydroxide, shellac, silicon dioxide, sodium lauryl sulphate, and titanium dioxide. The chemical name for rifabutin is 1',4-didehydro-1-deoxy-1,4-dihydro-5'-(2-methylpropyl)-1-oxorifamycin XIV (Chemical Abstracts Service, 9th Collective Index) or (9 S , 12 E , 14 S , 15 R , 16 S , 17 R , 18 R , 19 R , 20 S , 21 S , 22 E , 24 Z )-6,16,18,20-tetrahydroxy-1'-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-spiro [9,4-(epoxypentadeca[1,11,13]trienimino)-2 H- furo[2',3':7,8] naphth[1,2-d]imidazole-2,4'-piperidine]-5,10,26-(3 H ,9 H )-trione-16-acetate. Rifabutin has a molecular formula of C 46 H 62 N 4 O 11 , a molecular weight of 847.02 and the following structure: Rifabutin is a red-violet powder soluble in chloroform and methanol, sparingly soluble in ethanol, and very slightly soluble in water (0.19 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water). Chemical Structure
How Supplied
HOW SUPPLIED Rifabutin Capsules USP are supplied as size '0' capsules having opaque red-brown cap imprinted with 'LU' in white ink and opaque red-brown body imprinted with 'R01' in white ink, containing red-violet granular powder equivalent to 150 mg of rifabutin USP. Rifabutin Capsules USP are available as follows: Bottles of 60 capsules - NDC 68180-285-07 Bottles of 100 capsules - NDC 68180-285-01 Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature]. Keep tightly closed and dispense in a tight container as defined in the USP. Protect from light and from excessive heat. Manufactured for: Lupin Pharmaceuticals, Inc. Baltimore, Maryland 21202 United States Manufactured by: Lupin Limited Aurangabad 431 210 INDIA Revised: November 2021 ID#: 269014
Indications & Usage
INDICATIONS AND USAGE Rifabutin capsules USP are indicated for the prevention of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection.
Dosage and Administration
DOSAGE AND ADMINISTRATION It is recommended that rifabutin capsules USP be administered at a dose of 300 mg once daily. For those patients with propensity to nausea, vomiting, or other gastrointestinal upset, administration of rifabutin at doses of 150 mg twice daily taken with food may be useful. For patients with severe renal impairment (creatinine clearance less than 30 mL/min), consider reducing the dose of rifabutin by 50%, if toxicity is suspected. No dosage adjustment is required for patients with mild to moderate renal impairment. Reduction of the dose of rifabutin may also be needed for patients receiving concomitant treatment with certain other drugs (see Precautions - Drug Interactions ). Mild hepatic impairment does not require a dose modification. The pharmacokinetics of rifabutin in patients with moderate and severe hepatic impairment is not known.
