Drug Catalog - Product Detail
RIVASTIGMINE TARTRATE CP 1.5MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62756-0145-13 | SUN PHARMACEUTICALS | 500 | 1.5MG | CAPSULE |
PACKAGE FILES


Generic Name
RIVASTIGMINE TARTRATE
Substance Name
RIVASTIGMINE TARTRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077131
Description
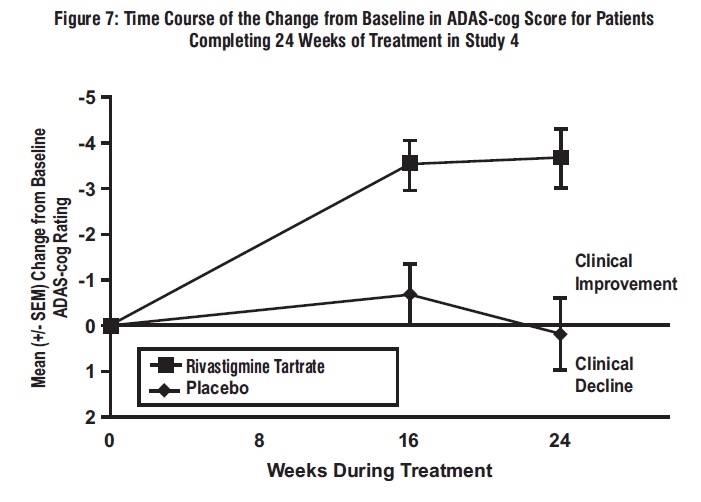
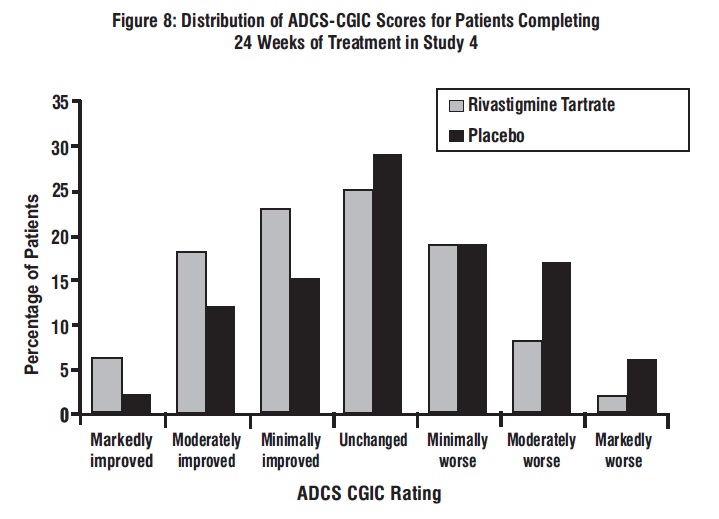
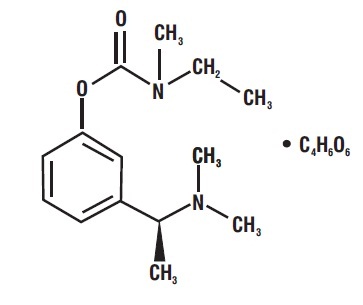
11 DESCRIPTION Rivastigmine tartrate is a reversible cholinesterase inhibitor and is known chemically as (S)-N-Ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate hydrogen-(2R,3R)-tartrate. Rivastigmine tartrate is commonly referred to in the pharmacological literature as SDZ ENA 713 or ENA 713. It has a molecular formula of C 14 H 22 N 2 O 2 • C 4 H 6 O 6 (hydrogen tartrate salt–hta salt) and a molecular weight of 400.43 g/mol (hta salt). Rivastigmine tartrate is a white to off-white, fine crystalline powder that is very soluble in water, soluble in ethanol and acetonitrile, slightly soluble in n-octanol and very slightly soluble in ethyl acetate. The distribution coefficient at 37°C in n-octanol/phosphate buffer solution pH 7 is 3. Rivastigmine tartrate capsules contain rivastigmine tartrate, USP, equivalent to 1.5 mg, 3 mg, 4.5 mg, and 6 mg of rivastigmine base for oral administration. Inactive ingredients are magnesium stearate, microcrystalline cellulose, and colloidal silicon dioxide. Each hard-gelatin capsule contains gelatin, titanium dioxide, D & C Red # 28 (1.5 mg), D & C Yellow # 10 (1.5 mg), FD & C Yellow # 6 (1.5 mg, 3 mg, 6 mg), FD & C Red # 40 (6 mg), red iron oxide (4.5 mg, 6 mg) and yellow iron oxide (4.5 mg, 6 mg). The imprinting ink contains shellac, dehydrated alcohol, butyl alcohol, propylene glycol, strong ammonia solution, potassium hydroxide and black iron oxide. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Rivastigmine Tartrate Capsules equivalent to 1.5 mg, 3 mg, 4.5 mg, or 6 mg of rivastigmine base are available as follows: 1.5 mg Capsule – yellow, “145” is printed in black on the body and cap of the capsule. Bottles of 60's with Child Resistant Cap.................................................... NDC 62756-145-86 Bottles of 500's with Non Child Resistant Cap.......................................... NDC 62756-145-13 Unit Dose (blister pack) Box of 100 (10x10)............................................. NDC 62756-145-61 3 mg Capsule – orange, “146” is printed in black on the body and cap of the capsule. Bottles of 60's with Child Resistant Cap.................................................... NDC 62756-146-86 Bottles of 500's with Non Child Resistant Cap.......................................... NDC 62756-146-13 Unit Dose (blister pack) Box of 100 (10x10)............................................. NDC 62756-146-61 4.5 mg Capsule – orange, “147” is printed in black on the body and cap of the capsule. Bottles of 60's with Child Resistant Cap..................................................... NDC 62756-147-86 Bottles of 500's with Non Child Resistant Cap........................................... NDC 62756-147-13 Unit Dose (blister pack) Box of 100 (10x10).............................................. NDC 62756-147-61 6 mg Capsule – orange, “148” is printed in black on the body and cap of the capsule. Bottles of 60's with Child Resistant Cap .................................................... NDC 62756-148-86 Bottles of 500's with Non Child Resistant Cap .......................................... NDC 62756-148-13 Unit Dose (blister pack) Box of 100 (10x10) ............................................. NDC 62756-148-61 Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Dispense in a tight container.
Indications & Usage
1 INDICATIONS AND USAGE Rivastigmine tartrate is an acetylcholinesterase inhibitor indicated for treatment of: Mild-to-moderate dementia of the Alzheimer’s type (AD) ( 1.1 ) Mild-to- moderate dementia associated with Parkinson’s disease (PD) ( 1.2 ) 1.1 Alzheimer's Disease Rivastigmine tartrate capsules are indicated for the treatment of mild-to-moderate dementia of the Alzheimer's type (AD). 1.2 Parkinson's Disease Dementia Rivastigmine tartrate capsules are indicated for the treatment of mild-to-moderate dementia associated with Parkinson’s disease (PD).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Alzheimer’s Disease (2.1): Initial Dose: Initiate treatment with 1.5 mg twice a day. Dose Titration: After a minimum of 2 weeks, if tolerated, increase dose to 3 mg twice a day and further to 4.5 mg twice a day and 6 mg twice a day if tolerated with a minimum of 2 weeks at each dose. Parkinson’s Disease Dementia (PDD) (2.2): Initial Dose: Initiate treatment with 1.5 mg twice a day Dose Titration: After a minimum of 4 weeks, if tolerated, increase dose to 3 mg twice a day and further to 4.5 mg twice a day and 6 mg twice a day if tolerated with a minimum of 4 weeks at each dose. Rivastigmine tartrate capsules should be taken with meals in divided doses in the morning and evening ( 2.1 , 2.2 ). Rivastigmine tartrate oral solution and rivastigmine tartrate capsules may be interchanged at equal doses ( 2.5 ). 2.1 Dosing in Alzheimer's Disease Rivastigmine tartrate capsules should be taken with meals in divided doses in the morning and evening. The recommended dosage of rivastigmine tartrate capsules in Alzheimer’s disease (AD) is 6 mg to 12 mg per day, administered twice a day (daily doses of 3 mg to 6 mg twice a day). There is evidence from the clinical trials that doses at the higher end of this range may be more beneficial. Initial Dose Initiate treatment with the 1.5 mg twice a day with rivastigmine tartrate capsules. Dose Titration After a minimum of 2 weeks and if well tolerated, increase the dose to 3 mg twice a day. Subsequent increases to 4.5 mg twice a day and 6 mg twice a day should be attempted after a minimum of 2 weeks at the previous dose and if well tolerated. The maximum dose is 6 mg twice a day (12 mg per day). 2.2 Dosing in Parkinson's Disease Dementia Rivastigmine tartrate capsules should be taken with meals in divided doses in the morning and evening. The dosage of rivastigmine tartrate capsules shown to be effective in the single controlled clinical trial conducted in dementia associated with Parkinson’s disease is 3 mg to 12 mg per day, administered twice a day (daily doses of 1.5 mg to 6 mg twice a day). Initial Dose Initiate treatment with the 1.5 mg twice a day with rivastigmine tartrate capsules. Dose Titration After a minimum of 4 weeks and if well tolerated, increase the dose to 3 mg twice a day. Subsequent increases to 4.5 mg twice a day and 6 mg twice a day should be attempted after a minimum of 4 weeks at the previous dose and if well tolerated. The maximum dose is 6 mg twice a day (12 mg per day). 2.3 Interruption of Treatment If adverse effects (e.g., nausea, vomiting, abdominal pain, loss of appetite) cause intolerance during treatment, the patient should be instructed to discontinue treatment for several doses and then restart at the same or next lower dose level. If dosing is interrupted for 3 days or fewer, restart treatment with the same or lower dose of rivastigmine tartrate capsules. If dosing is interrupted for more than 3 days, treatment should be restarted with 1.5 mg twice a day and titrated as described above [see Warnings and Precautions ( 5.1 )] . 2.4 Dosing in Specific Populations Dosing Modifications in Patients with Renal Impairment Patients with moderate and severe renal impairment may be able to only tolerate lower doses. Dosing Modifications in Patients with Hepatic Impairment Patients with mild (Child-Pugh score 5 to 6) and moderate (Child-Pugh score 7 to 9) hepatic impairment may be able to only tolerate lower doses. No data are available on the use of rivastigmine in patients with severe hepatic impairment. Dosing Modifications in Patients with Low Body Weight Carefully titrate and monitor patients with low body weight (less than 50 kg) for toxicities (e.g., excessive nausea, vomiting), and consider reducing the dose if such toxicities develop. 2.5 Important Administration Instructions Rivastigmine tartrate oral solution and rivastigmine tartrate capsules may be interchanged at equal doses.
