Drug Catalog - Product Detail
RIZATRIPTAN BENZOATE ODT TB 5MG 3X6 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0467-06 | GLENMARK PHARMACEUTICALS | 18 | 5MG | TABLET |
PACKAGE FILES






Generic Name
RIZATRIPTAN BENZOATE
Substance Name
RIZATRIPTAN BENZOATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA201914
Description
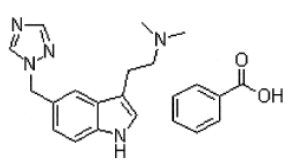
11 DESCRIPTION Rizatriptan benzoate orally disintegrating tablets, USP contain rizatriptan benzoate, USP a selective 5-hydroxytryptamine 1B/1D (5-HT 1B/1D ) receptor agonist. Rizatriptan benzoate, USP is described chemically as: N,N -dimethyl-5-(1 H -1,2,4-triazol-1-ylmethyl)-1 H -indole-3-ethanamine monobenzoate and its structural formula is: Its molecular formula is C 15 H 19 N 5 •C 7 H 6 O 2 , representing a molecular weight of the free base of 391.47 g/mol. Rizatriptan benzoate is a white to off-white powder that is soluble in methanol; sparingly soluble in water; slightly soluble in acetone and ethanol. Rizatriptan benzoate orally disintegrating tablets, USP are available for oral administration in strengths of 5 mg and 10 mg (corresponding to 7.265 mg or 14.53 mg of the benzoate salt, respectively). Each orally disintegrating tablet contains the following inactive ingredients: aspartame, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose and peppermint flavor. Meet USP Dissolution Test 2. structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Rizatriptan benzoate orally disintegrating tablets, USP 5 mg (equivalent to 7.265 mg rizatriptan benzoate) are white to off‑white, round, flat beveled edged, uncoated orally disintegrating tablets engraved with ‘467’ on one side and plain on the other side with a peppermint flavor. They are supplied as follows: Carton of 12 (2 x 6 unit-dose), NDC 68462-467-74 Carton of 18 (3 x 6 unit-dose), NDC 68462-467-06 Rizatriptan benzoate orally disintegrating tablets, USP 10 mg (equivalent to 14.53 mg rizatriptan benzoate) are white to off‑white, round, flat beveled edged, uncoated orally disintegrating tablets engraved with ‘468’ on one side and plain on the other side with a peppermint flavor. They are supplied as follows: Carton of 12 (2 x 6 unit-dose), NDC 68462-468-74 Carton of 18 (3 x 6 unit-dose), NDC 68462-468-06 Storage Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Indications & Usage
1 INDICATIONS AND USAGE Rizatriptan benzoate orally disintegrating tablets are indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years old. Limitations of Use • Rizatriptan benzoate orally disintegrating tablets should only be used where a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with rizatriptan benzoate orally disintegrating tablets, the diagnosis of migraine should be reconsidered before rizatriptan benzoate orally disintegrating tablets are administered to treat any subsequent attacks. • Rizatriptan benzoate orally disintegrating tablets are not indicated for use in the management of hemiplegic or basilar migraine [see Contraindications ( 4 )]. • Rizatriptan benzoate orally disintegrating tablets are not indicated for the prevention of migraine attacks. • Safety and effectiveness of rizatriptan benzoate orally disintegrating tablets have not been established for cluster headache. Rizatriptan benzoate is a serotonin (5-HT) 1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years of age ( 1 ) Limitations of Use: • Use only after clear diagnosis of migraine has been established ( 1 ) • Not indicated for the prophylactic therapy of migraine ( 1 ) • Not indicated for the treatment of cluster headache ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Adults: 5 mg or 10 mg single dose; separate repeat doses by at least two hours; maximum dose in a 24-hour period: 30 mg ( 2.1 ) • Pediatric patients 6 to 17 years: 5 mg single dose in patients less than 40 kg (88 lb); 10 mg single dose in patients 40 kg (88 lb) or more ( 2.2 ) • Adjust dose if co-administered with propranolol ( 2.4 ) 2.1 Dosing Information in Adults The recommended starting dose of rizatriptan benzoate orally disintegrating tablets is either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10 mg dose may provide a greater effect than the 5 mg dose, but may have a greater risk of adverse reactions [see Clinical Studies ( 14.1 )] . Redosing in Adults Although the effectiveness of a second dose or subsequent doses has not been established in placebo-controlled trials, if the migraine headache returns, a second dose may be administered 2 hours after the first dose. The maximum daily dose should not exceed 30 mg in any 24-hour period. The safety of treating, on average, more than four headaches in a 30-day period has not been established. 2.2 Dosing Information in Pediatric Patients (Age 6 to 17 Years) Dosing in pediatric patients is based on the patient's body weight. The recommended dose of rizatriptan benzoate orally disintegrating tablets is 5 mg in patients weighing less than 40 kg (88 lb), and 10 mg in patients weighing 40 kg (88 lb) or more. The efficacy and safety of treatment with more than one dose of rizatriptan benzoate orally disintegrating tablets within 24 hours in pediatric patients 6 to 17 years of age have not been established . 2.3 Administration of Rizatriptan Benzoate Orally Disintegrating Tablets For rizatriptan benzoate orally disintegrating tablets, administration with liquid is not necessary. Orally disintegrating tablets are packaged in a blister within a carton and patients should not remove the blister from the carton until just prior to dosing. The blister pack should then be peeled open with dry hands and the orally disintegrating tablet placed on the tongue, where it will dissolve and be swallowed with the saliva. 2.4 Dosage Adjustment for Patients on Propranolol Adult Patients In adult patients taking propranolol, only the 5 mg dose of rizatriptan benzoate orally disintegrating tablets is recommended, up to a maximum of 3 doses in any 24-hour period (15 mg) [see Drug Interactions ( 7.1 ) and Clinical Pharmacology ( 12.3 )]. Pediatric Patients For pediatric patients weighing 40 kg (88 lb) or more, taking propranolol, only a single 5-mg dose of rizatriptan benzoate orally disintegrating tablets is recommended (maximum dose of 5 mg in a 24-hour period). Rizatriptan benzoate orally disintegrating tablets should not be prescribed to propranolol-treated pediatric patients who weigh less than 40 kg (88 lb) [see Drug Interactions ( 7.1 ) and Clinical Pharmacology ( 12.3 )].
