Drug Catalog - Product Detail
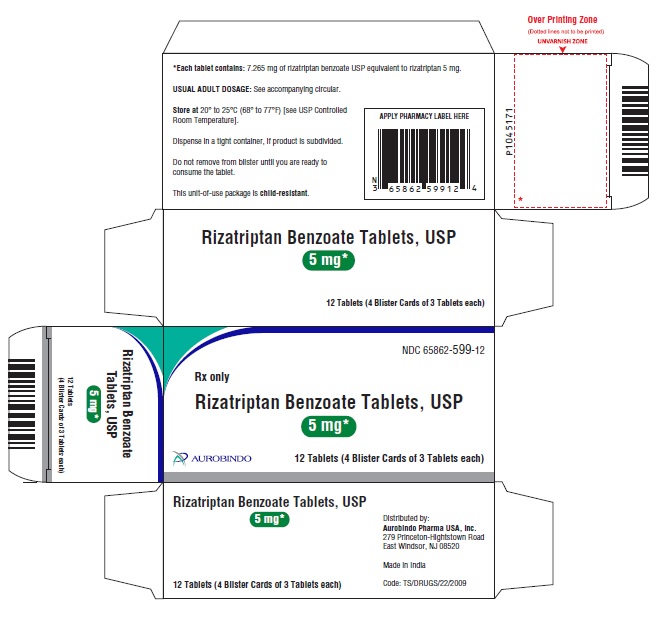
RIZATRIPTAN BENZOATE TAB 10mg 3X4 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0600-12 | AUROBINDO PHARMA | 12 | 10MG | TABLET |
PACKAGE FILES

Generic Name
RIZATRIPTAN BENZOATE
Substance Name
RIZATRIPTAN BENZOATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202490
Description
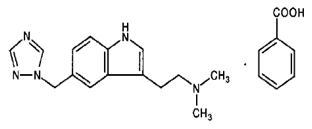
11 DESCRIPTION Rizatriptan benzoate tablets USP contain rizatriptan benzoate, a selective 5-hydroxytryptamine 1B/1D (5-HT 1B/1D ) receptor agonist. Rizatriptan benzoate is described chemically as: N,N -dimethyl-5-(1 H -1,2,4-triazol-1-ylmethyl)-1 H -indole-3-ethanamine monobenzoate and its structural formula is: Its molecular formula is C 15 H 19 N 5 •C 7 H 6 O 2 , representing a molecular weight of the free base of 269.35. Rizatriptan benzoate is a white to almost white crystalline powder that is soluble in water at about 42 mg per mL (expressed as free base) at 25°C. Rizatriptan benzoate tablets USP are available for oral administration in strengths of 5 mg and 10 mg (corresponding to 7.265 mg or 14.53 mg of the benzoate salt, respectively). Each compressed tablet contains the following inactive ingredients: corn starch, iron oxide red, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch (maize). Chemical Structure
How Supplied
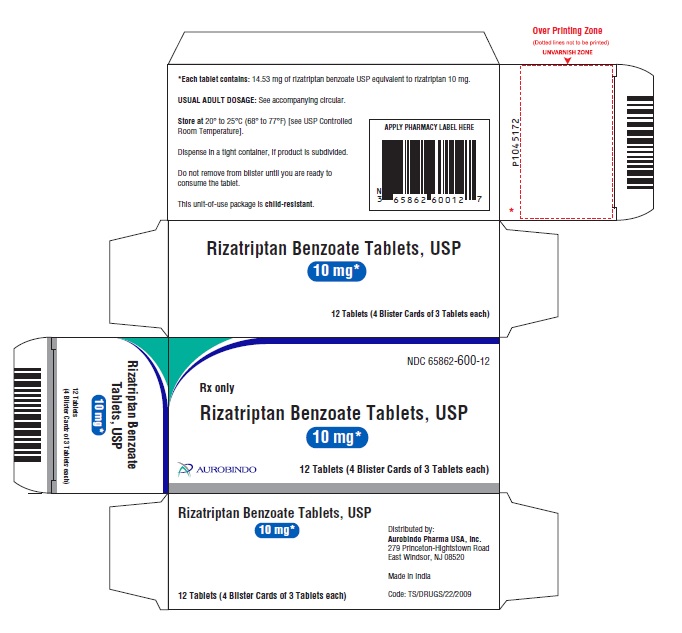
16 HOW SUPPLIED/STORAGE AND HANDLING Rizatriptan Benzoate Tablets USP, 5 mg are a pale pink-colored, circular, flat, beveled-edge uncoated tablets debossed with ‘X’ on one side and ‘13’ on other side. The tablets may be mottled. Unit-of-use blister package of 9 NDC 65862-599-90 Unit-of-use blister package of 12 NDC 65862-599-12 Unit-of-use blister package of 18 NDC 65862-599-18 Rizatriptan Benzoate Tablets USP, 10 mg are a pale pink-colored, circular, flat, beveled-edge uncoated tablets debossed with ‘X’ on one side and ‘14’ on other side. The tablets may be mottled. Unit-of-use blister package of 9 NDC 65862-600-90 Unit-of-use blister package of 12 NDC 65862-600-12 Unit-of-use blister package of 18 NDC 65862-600-18 Storage Store rizatriptan benzoate tablets USP at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Rizatriptan benzoate tablets are indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years old. Limitations of Use Rizatriptan benzoate tablets should only be used where a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with rizatriptan benzoate tablets, the diagnosis of migraine should be reconsidered before rizatriptan benzoate tablets are administered to treat any subsequent attacks. Rizatriptan benzoate tablets are not indicated for use in the management of hemiplegic or basilar migraine [see Contraindications (4) ] . Rizatriptan benzoate tablets are not indicated for the prevention of migraine attacks. Safety and effectiveness of rizatriptan benzoate tablets have not been established for cluster headache. Rizatriptan benzoate is a serotonin (5-HT) 1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years of age (1) Limitations of Use : Use only after clear diagnosis of migraine has been established (1) Not indicated for the prophylactic therapy of migraine (1) Not indicated for the treatment of cluster headache (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adults: 5 mg or 10 mg single dose; separate repeat doses by at least two hours; maximum dose in a 24-hour period: 30 mg (2.1) Pediatric patients 6 to 17 years: 5 mg single dose in patients less than 40 kg (88 lb); 10 mg single dose in patients 40 kg (88 lb) or more ( 2.2 ) Adjust dose if co-administered with propranolol (2.4) 2.1 Dosing Information in Adults The recommended starting dose of rizatriptan benzoate tablets is either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10 mg dose may provide a greater effect than the 5 mg dose, but may have a greater risk of adverse reactions [see Clinical Studies (14.1) ] . Redosing in Adults Although the effectiveness of a second dose or subsequent doses has not been established in placebo-controlled trials, if the migraine headache returns, a second dose may be administered 2 hours after the first dose. The maximum daily dose should not exceed 30 mg in any 24-hour period. The safety of treating, on average, more than four headaches in a 30-day period has not been established. 2.2 Dosing Information in Pediatric Patients (Age 6 to 17 Years) Dosing in pediatric patients is based on the patient's body weight. The recommended dose of rizatriptan benzoate tablets is 5 mg in patients weighing less than 40 kg (88 lb), and 10 mg in patients weighing 40 kg (88 lb) or more. The efficacy and safety of treatment with more than one dose of rizatriptan benzoate tablets within 24 hours in pediatric patients 6 to 17 years of age have not been established. 2.4 Dosage Adjustment for Patients on Propranolol Adult Patients In adult patients taking propranolol, only the 5 mg dose of rizatriptan benzoate tablets is recommended, up to a maximum of 3 doses in any 24-hour period (15 mg) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ]. Pediatric Patients For pediatric patients weighing 40 kg (88 lb) or more, taking propranolol, only a single 5 mg dose of rizatriptan benzoate tablets is recommended (maximum dose of 5 mg in a 24-hour period). Rizatriptan benzoate tablets should not be prescribed to propranolol-treated pediatric patients who weigh less than 40 kg (88 lb) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ].
