Drug Catalog - Product Detail
ROPINIROLE HCL TB 1MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43547-0270-50 | SOLCO HEALTHCARE | 500 | 1MG | TABLET |
PACKAGE FILES







Generic Name
ROPINIROLE HYDROCHLORIDE
Substance Name
ROPINIROLE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078110
Description
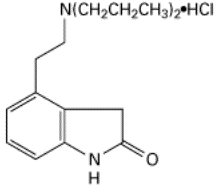
11 DESCRIPTION Ropinirole tablets contain ropinirole, a non-ergoline dopamine agonist, as the hydrochloride salt. The chemical name of ropinirole hydrochloride is 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and the empirical formula is C 16 H 24 N 2 OHCl. The molecular weight is 296.84 (260.38 as the free base). The structural formula is: Ropinirole hydrochloride is a white to yellow solid with a melting range of 243 o C to 250°C and a solubility of 133 mg/mL in water. Each round biconvex film-coated ropinirole tablet contains 0.29, 0.57, 1.14, 2.28, 3.42, 4.56, or 5.70 mg of ropinirole hydrochloride equivalent to ropinirole 0.25, 0.5, 1, 2, 3, 4, or 5 mg, respectively. Inactive ingredients of the core tablets consist of croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose. Inactive ingredients of the film coats are slightly different among the 7 strengths of tablets and are tabulated below: Strength Inactive ingredients of the film coat 0.25 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide and lecithin (soya). 0.5 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide and iron oxide yellow. 1 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide, FD&C Blue No. 2 aluminum lake and iron oxide yellow. 2 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide, lecithin (soya) and iron oxide red. 3 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide, carmine and FD&C Blue No. 1 aluminum lake. 4 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide, iron oxide yellow and iron oxide red. 5 mg polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, talc, titanium dioxide, FD&C Blue No. 2 aluminum lake and lecithin (soya). USP dissolution test is pending. structural formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Each round biconvex film-coated ropinirole tablet, USP, contains ropinirole hydrochloride equivalent to the labeled amount of ropinirole as follows: 0.25 mg: white tablets debossed “972” on one side and “HH” on the other side Bottles of 100 – NDC 43547-268-10 Bottles of 500 – NDC 43547-268-50 0.5 mg: yellow tablets debossed “973” on one side and “HH” on the other side Bottles of 100 – NDC 43547-269-10 Bottles of 500 – NDC 43547-269-50 1 mg: green tablets debossed “974” on one side and “HH” on the other side Bottles of 100 – NDC 43547-270-10 Bottles of 500 – NDC 43547-270-50 2 mg: pink tablets debossed “975” on one side and “HH” on the other side Bottles of 100 – NDC 43547-271-10 Bottles of 500 – NDC 43547-271-50 3 mg: purple tablets debossed “976” on one side and “HH” on the other side Bottles of 100 – NDC 43547-272-10 Bottles of 500 – NDC 43547-272-50 4 mg: beige tablets debossed “977” on one side and “HH” on the other side Bottles of 100 – NDC 43547-273-10 Bottles of 500 – NDC 43547-273-50 5 mg: blue tablets debossed “978” on one side and “HH” on the other side Bottles of 100 – NDC 43547-274-10 Bottles of 500 – NDC 43547-274-50 Storage Store at room temperature between 20°C and 25°C (68°F and 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light and moisture. Close container tightly after each use.
Indications & Usage
1 INDICATIONS AND USAGE Ropinirole tablets are a non-ergoline dopamine agonist indicated for the treatment of Parkinson’s disease (PD) and moderate-to-severe primary Restless Legs Syndrome (RLS). ( 1.1 , 1.2 ) 1.1 Parkinson’s Disease Ropinirole tablets are indicated for the treatment of Parkinson's disease. 1.2 Restless Legs Syndrome Ropinirole tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Ropinirole tablets can be taken with or without food. ( 2.1 ) • Retitration of ropinirole tablets may be warranted if therapy is interrupted. ( 2.1 ) Parkinson's Disease: • The recommended starting dose is 0.25 mg taken three times daily; titrate to a maximum daily dose of 24 mg. ( 2.2 ) • Renal Impairment: The maximum recommended dose is 18 mg/day in patients with end-stage renal disease on hemodialysis. ( 2.2 ) Restless Legs Syndrome: • The recommended starting dose is 0.25 mg once daily, 1 to 3 hours before bedtime, titrate to a maximum recommended dose of 4 mg daily. ( 2.3 ) • Renal Impairment: The maximum recommended dose is 3 mg/day in patients with end-stage renal disease on hemodialysis. ( 2.3 ) 2.1 General Dosing Recommendations Ropinirole tablets can be taken with or without food [see Clinical Pharmacology ( 12.3 )] . If a significant interruption in therapy with ropinirole tablets has occurred, retitration of therapy may be warranted. 2.2 Dosing for Parkinson’s Disease The recommended starting dose of ropinirole tablets for Parkinson's disease is 0.25 mg 3 times daily. Based on individual patient therapeutic response and tolerability, if necessary, the dose should then be titrated with weekly increments as described in Table 1. After Week 4, if necessary, the daily dose may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly up to a maximum recommended total daily dose of 24 mg/day (8 mg 3 times daily). Doses greater than 24 mg/day have not been tested in clinical trials. Table 1. Ascending-Dose Schedule of Ropinirole Tablets for Parkinson’s Disease Week Dosage Total Daily Dose 1 0.25 mg 3 times daily 0.75 mg 2 0.5 mg 3 times daily 1.5 mg 3 0.75 mg 3 times daily 2.25 mg 4 1 mg 3 times daily 3 mg Ropinirole tablets should be discontinued gradually over a 7-day period in patients with Parkinson's disease. The frequency of administration should be reduced from 3 times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of ropinirole tablets. Renal Impairment No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg 3 times a day. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole tablets in patients with severe renal impairment without regular dialysis has not been studied. 2.3 Dosing for Restless Legs Syndrome The recommended adult starting dose for RLS is 0.25 mg once daily 1 to 3 hours before bedtime. After 2 days, if necessary, the dose can be increased to 0.5 mg once daily, and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 2 as needed to achieve efficacy. Titration should be based on individual patient therapeutic response and tolerability, up to a maximum recommended dose of 4 mg daily. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. Table 2. Dose Titration Schedule of Ropinirole Tablets for Restless Legs Syndrome Day/Week Dose to be taken once daily 1 to 3 hours before bedtime Days 1 and 2 0.25 mg Days 3 to 7 0.5 mg Week 2 1 mg Week 3 1.5 mg Week 4 2 mg Week 5 2.5 mg Week 6 3 mg Week 7 4 mg When discontinuing ropinirole tablets in patients with RLS, gradual reduction of the daily dose is recommended [see Warnings and Precautions ( 5.8 , 5.9 )]. Renal Impairment No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg once daily. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 3 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole tablets in patients with severe renal impairment without regular dialysis has not been studied.
