Drug Catalog - Product Detail
ROPINIROLE HCL TB 3MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-5503-01 | MYLAN | 100 | 3MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
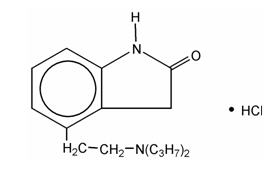
DESCRIPTION Ropinirole hydrochloride is an orally administered non-ergoline dopamine agonist. It is the hydrochloride salt of 4-[2-(dipropylamino)-ethyl]-1,3-dihydro-2 H -indol-2-one and has an molecular formula of C 16 H 24 N 2 O•HCl. The molecular weight is 296.84 (260.38 as the free base). The structural formula is: Ropinirole hydrochloride is a white to cream colored crystalline powder with a melting range of 241° to 245°C and a solubility of 133 mg/mL in water. Each tablet contains ropinirole hydrochloride equivalent to ropinirole, 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg or 5 mg. Inactive ingredients consist of: anhydrous lactose, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and microcrystalline cellulose. In addition, the following product specific coloring agents are employed: 0.5 mg - D and C Yellow No. 10 Aluminum Lake 1 mg - D and C Yellow No. 10 Aluminum Lake, FD and C Blue No. 2 Aluminum Lake 2 mg - D and C Yellow No. 10 Aluminum Lake, FD and C Blue No. 2 Aluminum Lake, FD and C Yellow No. 6 Aluminum Lake 3 mg - D and C Yellow No. 10 Aluminum Lake, FD and C Blue No. 2 Aluminum Lake, FD and C Yellow No. 6 Aluminum Lake, FD and C Red No. 40 Aluminum Lake 4 mg - D and C Yellow No. 10 Aluminum Lake, FD and C Blue No. 2 Aluminum Lake, FD and C Yellow No. 6 Aluminum Lake, FD and C Red No. 40 Aluminum Lake 5 mg - D and C Yellow No. 10 Aluminum Lake, FD and C Blue No. 2 Aluminum Lake, FD and C Yellow No. 6 Aluminum Lake, FD and C Red No. 40 Aluminum Lake Structure Image
How Supplied
HOW SUPPLIED Ropinirole Hydrochloride Tablets are available containing 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg or 5 mg of ropinirole hydrochloride. The 0.25 mg tablets are white, round unscored tablets debossed with M on one side of the tablet and N over 25 on the other side. They are available as follows: NDC 0378-5525-01 bottles of 100 tablets NDC 0378-5525-05 bottles of 500 tablets The 0.5 mg tablets are yellow, round unscored tablets debossed with M on one side of the tablet and N over 5 on the other side. They are available as follows: NDC 0378-5550-01 bottles of 100 tablets NDC 0378-5550-05 bottles of 500 tablets The 1 mg tablets are green, round unscored tablets debossed with M on one side of the tablet and N over 10 on the other side. They are available as follows: NDC 0378-5501-01 bottles of 100 tablets NDC 0378-5501-05 bottles of 500 tablets The 2 mg tablets are orange, round unscored tablets debossed with M on one side of the tablet and N over 20 on the other side. They are available as follows: NDC 0378-5502-01 bottles of 100 tablets NDC 0378-5502-05 bottles of 500 tablets The 3 mg tablets are lavender, round unscored tablets debossed with M on one side of the tablet and N over 30 on the other side. They are available as follows: NDC 0378-5503-01 bottles of 100 tablets NDC 0378-5503-05 bottles of 500 tablets The 4 mg tablets are grayish beige, round unscored tablets debossed with M on one side of the tablet and N over 40 on the other side. They are available as follows: NDC 0378-5504-01 bottles of 100 tablets NDC 0378-5504-05 bottles of 500 tablets The 5 mg tablets are blue, round unscored tablets debossed with M on one side of the tablet and N over 50 on the other side. They are available as follows: NDC 0378-5505-01 bottles of 100 tablets NDC 0378-5505-05 bottles of 500 tablets Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. PHARMACIST: Dispense a Patient Information Leaflet with each prescription. Mylan Pharmaceuticals Inc. Morgantown, WV 26505 REVISED AUGUST 2009 RPNL:R2ppbpt
Indications & Usage
INDICATIONS AND USAGE Parkinson's Disease Ropinirole hydrochloride tablets are indicated for the treatment of the signs and symptoms of idiopathic Parkinson's disease. The effectiveness of ropinirole hydrochloride tablets was demonstrated in randomized, controlled trials in patients with early Parkinson's disease who were not receiving concomitant L-dopa therapy as well as in patients with advanced disease on concomitant L-dopa (see CLINICAL PHARMACOLOGY: Clinical Trials ). Restless Legs Syndrome Ropinirole hydrochloride tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS). Key diagnostic criteria for RLS are: an urge to move the legs usually accompanied or caused by uncomfortable and unpleasant leg sensations; symptoms begin or worsen during periods of rest or inactivity such as lying or sitting; symptoms are partially or totally relieved by movement such as walking or stretching at least as long as the activity continues; and symptoms are worse or occur only in the evening or night. Difficulty falling asleep may frequently be associated with moderate-to-severe RLS.
Dosage and Administration
DOSAGE AND ADMINISTRATION General Dosing Considerations for Parkinson's Disease and RLS Ropinirole hydrochloride tablets can be taken with or without food. Patients may be advised that taking ropinirole hydrochloride tablets with food may reduce the occurrence of nausea. However, this has not been established in controlled clinical trials. If a significant interruption in therapy with ropinirole hydrochloride tablets has occurred, retitration of therapy may be warranted. Geriatric Use Pharmacokinetic studies demonstrated a reduced clearance of ropinirole in the elderly (see CLINICAL PHARMACOLOGY ). Dose adjustment is not necessary since the dose is individually titrated to clinical response. Renal Impairment The pharmacokinetics of ropinirole were not altered in patients with moderate renal impairment (see CLINICAL PHARMACOLOGY ). Therefore, no dosage adjustment is necessary in patients with moderate renal impairment. The use of ropinirole hydrochloride tablets in patients with severe renal impairment has not been studied. Hepatic Impairment The pharmacokinetics of ropinirole have not been studied in patients with hepatic impairment. Since patients with hepatic impairment may have higher plasma levels and lower clearance, ropinirole hydrochloride tablets should be titrated with caution in these patients. Dosing for Parkinson's Disease In all clinical studies, dosage was initiated at a subtherapeutic level and gradually titrated to therapeutic response. The dosage should be increased to achieve a maximum therapeutic effect, balanced against the principal side effects of nausea, dizziness, somnolence, and dyskinesia. The recommended starting dose for Parkinson's disease is 0.25 mg 3 times daily. Based on individual patient response, dosage should then be titrated with weekly increments as described in Table 5. After week 4, if necessary, daily dosage may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly to a total dose of 24 mg/day. Doses greater than 24 mg/day have not been tested in clinical trials. Table 5. Ascending-Dose Schedule of Ropinirole Hydrochloride Tablets for Parkinson's Disease Week Dosage Total Daily Dose 1 0.25 mg 3 times daily 0.75 mg 2 0.5 mg 3 times daily 1.5 mg 3 0.75 mg 3 times daily 2.25 mg 4 1 mg 3 times daily 3 mg When ropinirole hydrochloride tablets are administered as adjunct therapy to L-dopa, the concurrent dose of L-dopa may be decreased gradually as tolerated. L-dopa dosage reduction was allowed during the advanced Parkinson's disease (with L-dopa) study if dyskinesias or other dopaminergic effects occurred. Overall, reduction of L-dopa dose was sustained in 87% of patients treated with ropinirole hydrochloride tablets and in 57% of patients on placebo. On average the L-dopa dose was reduced by 31% in patients treated with ropinirole hydrochloride tablets. Ropinirole hydrochloride tablets for Parkinson's disease patients should be discontinued gradually over a 7 day period. The frequency of administration should be reduced from 3 times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of ropinirole hydrochloride tablets. Dosing for Restless Legs Syndrome In all clinical trials, the dose for ropinirole hydrochloride tablets was initiated at 0.25 mg once daily, 1 to 3 hours before bedtime. Patients were titrated based on clinical response and tolerability. The recommended adult starting dosage for RLS is 0.25 mg once daily, 1 to 3 hours before bedtime. After 2 days, the dosage can be increased to 0.5 mg once daily and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 6 as needed to achieve efficacy. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. Table 6. Dose Titration Schedule for RLS Day/Week Dosage to be taken once daily, 1 to 3 hours before bedtime. Days 1 and 2 0.25 mg Days 3 to 7 0.5 mg Week 2 1 mg Week 3 1.5 mg Week 4 2 mg Week 5 2.5 mg Week 6 3 mg Week 7 4 mg In clinical trials of patients being treated for RLS with doses up to 4 mg once daily, ropinirole hydrochloride tablets was discontinued without a taper.
