Drug Catalog - Product Detail
SCOPOLAMINE TRANSDERMAL SYSTEM 1MG PATCH 24CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0580-62 | PADAGIS | 24 | 1MG/3DAYS | TRANSDERMAL SYSTEM |
PACKAGE FILES


Generic Name
SCOLOPAMINE TRANSDERMAL SYSTEM
Substance Name
SCOPOLAMINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
ANDA078830
Description
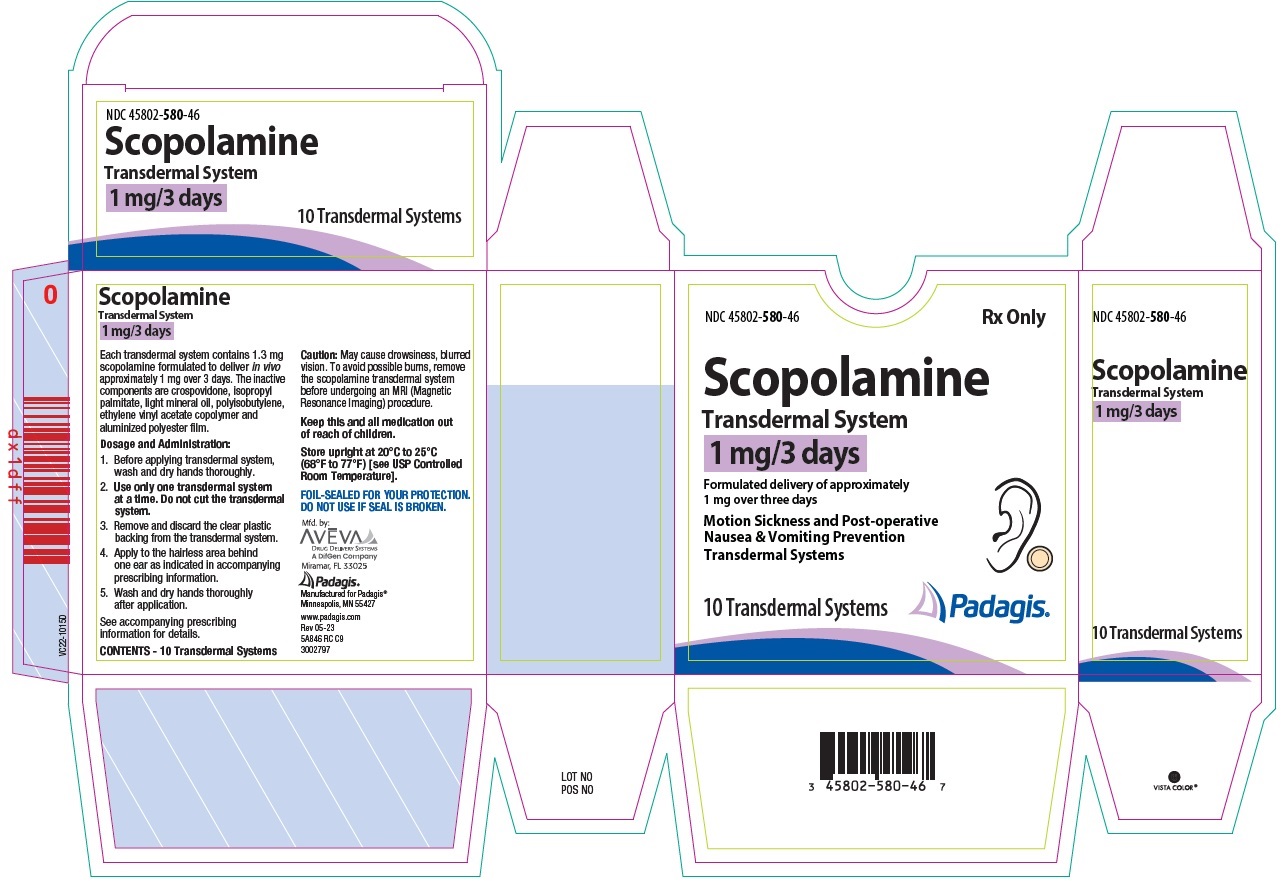
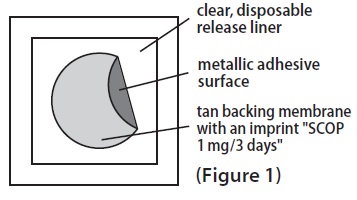
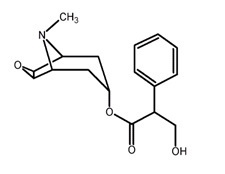
11 DESCRIPTION Scopolamine transdermal system is designed for continuous release of scopolamine following application to an area of intact skin on the head, behind the ear. Each system contains 1.3 mg of scopolamine base. Scopolamine is (9-methyl-3-oxa-9-azatricyclo[3.3.1.0 2,4 ]nonan-7-yl) 3-hydroxy-2-phenylpropanoate. The empirical formula is C 17 H 21 NO 4 and its structural formula is: Scopolamine has a molecular weight of 303.35 and a pKa of 7.55-7.81. The scopolamine transdermal system is a circular, 0.2 mm thick, 2.5 cm 2 film with four layers. Proceeding from the visible surface towards the surface attached to the skin, these layers are: (1) a backing membrane of tan-colored, aluminized, polyester film; (2) a drug layer of scopolamine, crospovidone, isopropyl palmitate, light mineral oil, and polyisobutylene; (3) an ethylene vinyl acetate copolymer membrane that controls the rate of delivery of scopolamine from the system to the skin surface; and (4) an contact layer formulation of crospovidone, isopropyl palmitate, light mineral oil, polyisobutylene, and scopolamine. A release liner of siliconized polyester, which covers the adhesive layer, is removed before the system is used. Cross section of the system: structure cross-section
How Supplied
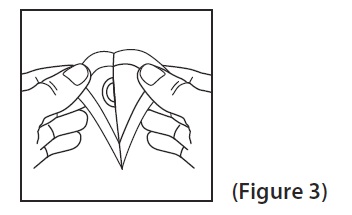
16 HOW SUPPLIED/STORAGE AND HANDLING Scopolamine Transdermal System 1 mg/3 days is available as the following: Cartons of 4, 10, and 24 transdermal systems, packaged into individual foil pouches. • Carton of 4 transdermal systems. NDC 45802-580-84 • Carton of 10 transdermal systems. NDC 45802-580-46 • Carton of 24 transdermal systems. NDC 45802-580-62 Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Store pouch(es) in an upright position. Do not bend or roll pouch(es). Wash hands thoroughly with soap and water immediately after handling the transdermal system. Avoid touching the system during the treatment. Upon removal, fold the used transdermal system in half with the sticky side together, and discard in household trash in a manner that prevents accidental contact or ingestion by children, pets or others. Wash the hands and application site with soap and water after transdermal system removal [see Dosage and Administration ( 2.1 ), Warnings and Precautions ( 5.6 )] .
Indications & Usage
1 INDICATIONS AND USAGE Scopolamine transdermal system is indicated in adults for the prevention of: • nausea and vomiting associated with motion sickness. • post-operative nausea and vomiting (PONV) associated with recovery from anesthesia and/or opiate analgesia and surgery. Scopolamine transdermal system is an anticholinergic indicated in adults for the prevention of: • nausea and vomiting associated with motion sickness. ( 1 ) • post-operative nausea and vomiting (PONV) associated with recovery from anesthesia and/or opiate analgesia and surgery. ( 1 )
Dosage and Administration
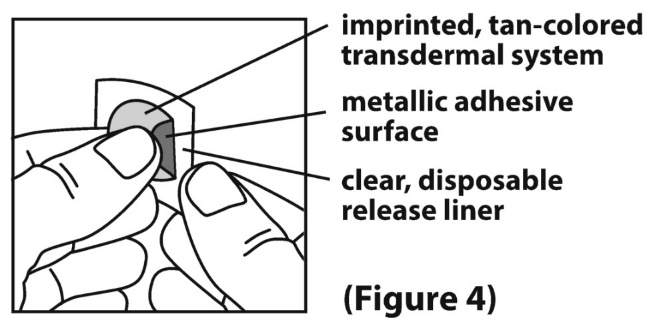
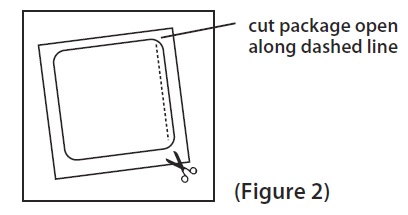
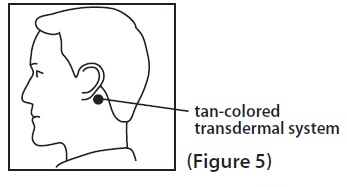
2 DOSAGE AND ADMINISTRATION Application and Removal ( 2.1 ): • Each scopolamine transdermal system delivers 1 mg of scopolamine over 3 days. • Only wear one transdermal system at a time. • Do not cut the transdermal system. • Wash hands thoroughly with soap and water after application. • Avoid touching or applying pressure to the transdermal system once applied. • Upon removal, fold used transdermal system in half with sticky side together, discard to prevent accidental contact or ingestion, and wash the hands and application site with soap and water. Recommended Dosage : • Motion Sickness : Apply one transdermal system to the hairless area behind one ear at least 4 hours before antiemetic effect is required for use up to 3 days. If therapy for more than 3 days is required, remove the first transdermal system and apply a new transdermal system behind the other ear. ( 2.2 ) • PONV : For surgeries other than cesarean section, apply one transdermal system behind the ear the evening before surgery and remove 24 hours following surgery. ( 2.2 ) 2.1 Important Application and Removal Instructions • Each scopolamine transdermal system is formulated to deliver in vivo approximately 1 mg of scopolamine over 3 days. • Only wear one transdermal system at any time. • Do not cut the transdermal system. • Apply the transdermal system to the skin in the postauricular area (hairless area behind one ear). • After the transdermal system is applied on the dry skin behind the ear, wash hands thoroughly with soap and water and dry hands [see Warnings and Precautions ( 5.6 )] . • If the transdermal system becomes displaced, discard the transdermal system, and apply a new transdermal system on the hairless area behind the other ear. • Once the transdermal system has been affixed, avoid touching or applying pressure to the transdermal system while it is being worn, since pressure exerted on it may cause scopolamine to ooze out at the edge. • Upon removal, fold the used transdermal system in half with the sticky side together, and discard in household trash in a manner that prevents accidental contact or ingestion by children, pets or others. • Wash the hands and application site with soap and water after transdermal system removal [see Warnings and Precautions ( 5.6 )] . 2.2 Recommended Adult Dosage Motion Sickness Apply one scopolamine transdermal system to the hairless area behind one ear at least 4 hours before the antiemetic effect is required – for use up to 3 days. If therapy is required for longer than 3 days, remove the first transdermal system and apply a new scopolamine transdermal system behind the other ear. PONV For surgeries other than cesarean section : Apply one scopolamine transdermal system the evening before scheduled surgery. Remove the transdermal system 24 hours following surgery.
