Drug Catalog - Product Detail
Silodosin Cap 8 MG 30 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 31722-0636-30 | CAMBER PHARMACEUTICALS | 30 | 8MG | CAPSULE |
PACKAGE FILES





Generic Name
SILODOSIN
Substance Name
SILODOSIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204793
Description
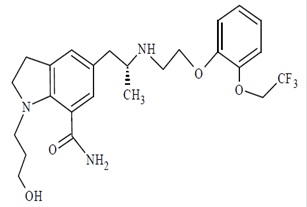
11 DESCRIPTION Silodosin is a selective antagonist of alpha-1 adrenoreceptors. The chemical name of Silodosin is 2,3-Dihydro-1-(3-hydroxy-propyl)-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy) phenoxy] ethyl]amino]-propyl]-1H-indole-7-carboxamide and the molecular formula is C 25 H 32 F 3 N 3 O 4 with a molecular weight of 495.55. The structural formula of silodosin is: Silodosin is a white to pale yellowish white powder. It is sparingly soluble in methanol. Each silodosin capsule for oral administration contains 4 mg or 8 mg of silodosin, and the following inactive ingredients: gelatin, pregelatinized starch, sodium stearyl fumarate, sorbitol and titanium dioxide. The imprinting ink containing black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac and strong ammonia solution. stucture
How Supplied
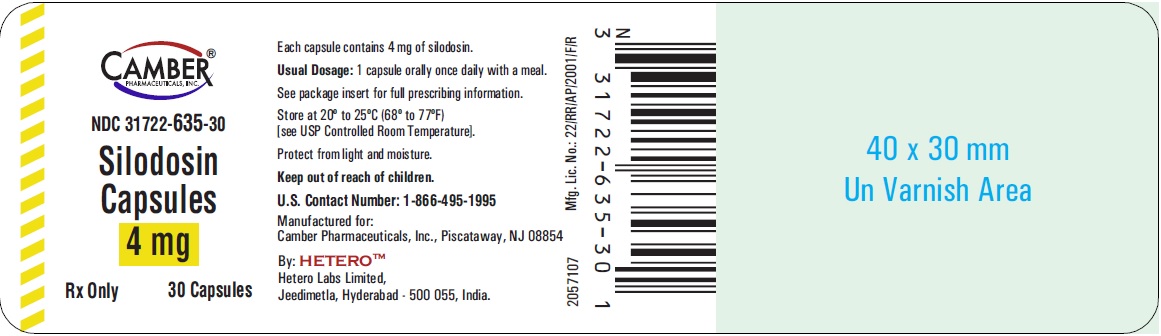
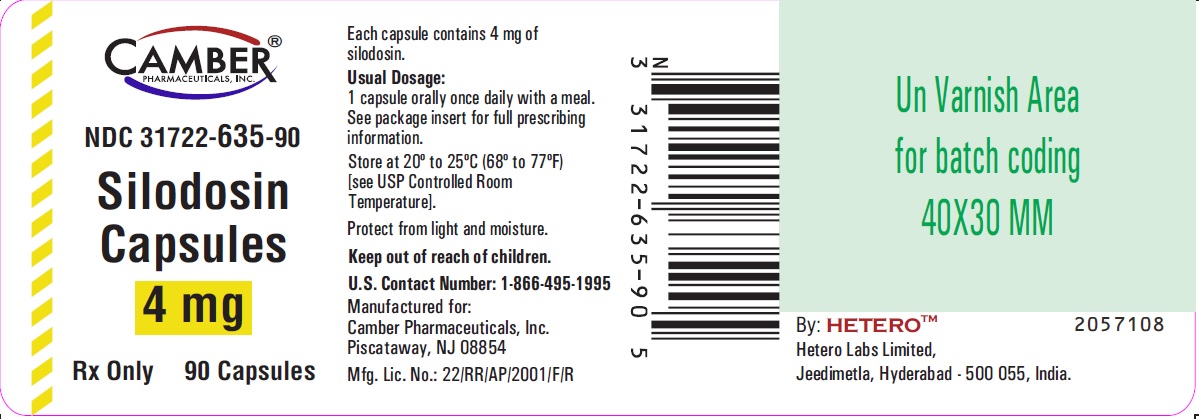
16 HOW SUPPLIED/STORAGE AND HANDLING Silodosin Capsules, 4 mg are White cap / White body size ‘3’ hard gelatin capsules imprinted with ‘H’ on cap and ‘S1’ on body, filled with white to off white powder. They are supplied as follows: White Colored Bottles of 30 Capsules NDC 31722-635-30 Amber Colored Bottles of 30 Capsules NDC 31722-635-31 White Colored Bottles of 90 Capsules NDC 31722-635-90 Amber Colored Bottles of 90 Capsules NDC 31722-635-91 Bottles of 30 and 90 capsules are supplied with child-resistant closures. Silodosin Capsules, 8 mg are White cap / White body size ‘1’ hard gelatin capsules imprinted with ‘H’ on cap and ‘S2’ on body, filled with white to off white powder. They are supplied as follows: White Colored Bottles of 30 Capsules NDC 31722-636-30 Amber Colored Bottles of 30 Capsules NDC 31722-636-31 White Colored Bottles of 90 Capsules NDC 31722-636-90 Amber Colored Bottles of 90 Capsules NDC 31722-636-91 Bottles of 30 and 90 capsules are supplied with child-resistant closures. Storage Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light and moisture. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Silodosin capsules, a selective alpha-1 adrenergic receptor antagonist, is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) [see CLINICAL STUDIES ( 14 )]. Silodosin capsules are not indicated for the treatment of hypertension. Silodosin capsules, an alpha-1 adrenergic receptor antagonist, is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH). Silodosin capsules are not indicated for the treatment of hypertension. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • 8 mg capsules taken orally once daily with a meal. ( 2.1 ) • 4 mg capsules taken orally once daily with a meal for those with moderate renal impairment [Creatinine Clearance (CCr) 30 to 50 mL/min]. ( 2.2 ) 2.1 Dosing Information The recommended dose is 8 mg orally once daily with a meal. Patients who have difficulty swallowing pills and capsules may carefully open the silodosin capsule and sprinkle the powder inside on a tablespoonful of applesauce. The applesauce should be swallowed immediately (within 5 minutes) without chewing and followed with an 8 oz glass of cool water to ensure complete swallowing of the powder. The applesauce used should not be hot, and it should be soft enough to be swallowed without chewing. Any powder/applesauce mixture should be used immediately (within 5 minutes) and not stored for future use. Subdividing the contents of a silodosin capsule is not recommended [see CLINICAL PHARMACOLOGY ( 12.3 )]. 2.2 Dosage Adjustment in Special Populations Renal impairment: Silodosin capsules are contraindicated in patients with severe renal impairment (CCr < 30 mL/min). In patients with moderate renal impairment (CCr 30 to 50 mL/min), the dose should be reduced to 4 mg once daily taken with a meal. No dosage adjustment is needed in patients with mild renal impairment (CCr 50 to 80 mL/min) [see CONTRAINDICATIONS ( 4 ), WARNINGS AND PRECAUTIONS ( 5.2 ), USE IN SPECIFIC POPULATIONS ( 8.6 ) and CLINICAL PHARMACOLOGY ( 12.3 )]. Hepatic impairment: Silodosin capsules have not been studied in patients with severe hepatic impairment (Child-Pugh score ≥ 10) and is therefore contraindicated in these patients. No dosage adjustment is needed in patients with mild or moderate hepatic impairment [see CONTRAINDICATIONS ( 4 ), WARNINGS AND PRECAUTIONS ( 5.3 ), USE IN SPECIFIC POPULATIONS ( 8.7 ) and CLINICAL PHARMACOLOGY ( 12.3 )].
